Background
Little is known about racial-ethnic differences in the distribution of maternal serum levels of angiogenic and antiangiogenic factors and their associations with early-onset preeclampsia.
Objective
We sought to investigate the distribution of midtrimester maternal serum levels of placental growth factor, soluble endoglin, and soluble vascular endothelial growth factor receptor 1 and their associations with early-onset preeclampsia in whites, Hispanics, and blacks.
Study Design
A population-based nested case-control design was used to identify cases and controls of white, Hispanic, and black origin from a 2000 through 2007 live-birth cohort in 5 southern California counties. Cases included 197 women (90 whites, 67 Hispanics, and 40 blacks) with early-onset preeclampsia defined as hypertension and proteinuria with onset <32 weeks according to hospital records. Controls included a random sample of 2363 women without early-onset preeclampsia. Maternal serum specimens collected at 15-20 weeks’ gestation as part of routine prenatal screening were tested for placental growth factor, soluble endoglin, and soluble vascular endothelial growth factor receptor 1. Serum levels of the 3 factors were log-normally distributed. Adjusted natural logarithmic means were compared between cases and controls and between racial-ethnic groups. Odds ratios and 95% confidence intervals derived from logistic regression models were calculated to measure the magnitude of the associations.
Results
Cases showed lower adjusted logarithmic means of placental growth factor but higher adjusted logarithmic means of soluble endoglin than controls across all 3 groups ( P < .05). Cases also had higher adjusted means of soluble vascular endothelial growth factor receptor 1 than controls in whites (7.75 vs 7.52 log pg/mL, P < .05) and Hispanics (7.73 vs 7.40 log pg/mL, P < .05) but not in blacks (7.85 vs 7.69 log pg/mL, P = .47). Blacks were found to have higher levels of placental growth factor in both cases and controls when compared to whites and Hispanics (adjusted means: 4.69 and 5.20 log pg/mL in blacks, 4.08 and 4.78 log pg/mL in whites, and 3.89 and 4.70 log pg/mL in Hispanics, respectively, P < .05). Hispanic cases had the highest adjusted mean of soluble endoglin compared to white and black cases (9.24, 9.05, and 8.93 log pg/mL, respectively, P < .05). The weakest association of early-onset preeclampsia with placental growth factor and soluble endoglin was observed in blacks. The adjusted odds ratio per log pg/mL increase of the 2 analytes were 0.219 (95% confidence interval, 0.124–0.385) and 5.02 (95% confidence interval, 2.56–9.86) in blacks in comparison to 0.048 (95% confidence interval, 0.026–0.088) and 36.87 (95% confidence interval, 17.00–79.96) in whites ( P < .05) and 0.028 (95% confidence interval, 0.013–0.060) and 86.68 (95% confidence interval, 31.46–238.81) in Hispanics ( P < .05), respectively. As for soluble vascular endothelial growth factor receptor 1, the association was not significantly different among the racial-ethnic groups.
Conclusion
Racial-ethnic differences were observed in the distribution of midtrimester maternal levels of placental growth factor and soluble endoglin and in the associations with early-onset preeclampsia. These differences should be considered in future studies to improve etiologic and prognostic understanding of early-onset preeclampsia.
Introduction
Preeclampsia is one of the most dangerous pregnancy-associated disorders. Affecting 3-5% of pregnancies in the United States, preeclampsia causes 25% of maternal deaths and 25,000-40,000 perinatal deaths each year. Preeclampsia is heterogeneous in onset and severity of clinical manifestations. Preeclampsia occurring at <32 weeks of pregnancy, also known as early-onset preeclampsia with an incidence range of 0.2-1.4%, carries a disproportionately higher risk of maternal and infant mortality and therefore is considered a more severe form of the disease than preeclampsia occurring later in pregnancy. Angiogenesis is the process of new blood vessel formation and the growth of new blood vessels from existing blood vessels. These processes are critical to the establishment and functioning of the placenta. The placenta produces high levels of angiogenic factors such as placental growth factor (PlGF) to maintain an angiogenic balance for normal fetal development. Dramatically reduced levels of PlGF and increased levels of antiangiogenic factors such as soluble endoglin (sEng) and soluble vascular endothelial growth factor receptor (sVEGFR)-1 in maternal circulation have been repeatedly reported as important predictors for preeclampsia with more pronounced effects for early-onset compared to late-onset preeclampsia.
Black women have been shown to be at higher risk for preeclampsia than white women (4.0% vs 2.8%). Hispanics have been found to have lower risk than non-Hispanics (1.3% vs 5.3%) although results are not consistent. While many studies have been published on angiogenic and antiangiogenic factors as screening markers for preeclampsia, few have examined racial-ethnic differences in the distribution of these factors and in their associations with preeclampsia. To date, no population-based study that we are aware of has concurrently compared the pattern of mid-pregnancy angiogenic and antiangiogenic factors in white, Hispanic, and black women. A comprehensive study of racial-ethnic differences in maternal angiogenic imbalance is needed to better understand the pathogenesis of preeclampsia within and across racial-ethnic groups.
Utilizing a nested case-control study design, this study aimed to better understand the relationship among maternal serum concentrations of PlGF, sEng, and sVEGFR-1 at 15-20 weeks’ gestation in white, Hispanic, and black women with and without early-onset preeclampsia.
Materials and Methods
Case identification
All early-onset preeclampsia cases (n = 197) evaluated in this study were identified by the California Very Preterm Birth Study from prenatal screening records and validated via chart review at the hospital of delivery. Detailed study design and methods have been published elsewhere. Briefly, very preterm births of white, Hispanic, or black origin were identified from a population-based cohort created through record linkage of 346,456 mother-baby pairs with stored maternal serum specimens linked to certificates of live births in California from January 2000 through April 2007. White and Hispanic births were defined as both parents being non-Hispanic white and Mexican Hispanic white, respectively, based on birth records. Mexican Hispanic white referred to self-reported white with Hispanic ethnicity origin of Mexican, Mexican-American, or Chicano. Black births were defined as black mothers of any ethnicity, regardless of father’s race-ethnicity. All of these women participated in routine prenatal screening for aneuploidies and neural tube defects between 15-20 weeks through the Genetic Disease Screening Program within the California Department of Public Health and had serum banked after screening. Study samples were limited to singleton live births delivered in 5 southern California counties (Los Angeles, Orange, Riverside, San Bernardino, and San Diego). Within this large cohort, the medical records of 1117 chronologically identified very preterm births of <32 weeks gestation (429 whites, 440 Hispanics, and 248 blacks) were abstracted to distinguish very preterm births with known causes including preeclampsia, which formed the case group for this study. Cases with a birthweight >2500 g were excluded based on suspected misdating. Pregnancies with structural abnormalities reported on delivery records or identified by the California Birth Defects Monitoring Program registry were also excluded.
Preeclampsia was defined as hypertension accompanied by proteinuria with onset >20 weeks’ gestation in the California Very Preterm Birth Study. Hypertension was defined as blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic on 2 occasions 6 hours apart. Proteinuria was defined as ≥0.3 g of protein in a 24-hour urine collection or ≥1+ dipstick on a urine test on 2 occasions 6 hours apart. Early-onset preeclampsia was defined as preeclampsia with delivery <32 weeks’ gestation. Gestational age estimates for cases were mostly based on ultrasound (61.5%) and date of last menstrual period (29.5%) from the California Prenatal Screening Program with a small part (9.0%) based on maternal hospital chart review.
Hospital chart review identified 207 cases of early-onset preeclampsia with a maternal midtrimester specimen available for laboratory testing. Ten of the 207 cases were excluded due to maternal specimens collected beyond the gestational window of 15-20 weeks. The final sample included 90 white, 67 Hispanic, and 40 black cases. Details of the sample selection are listed in the Figure .
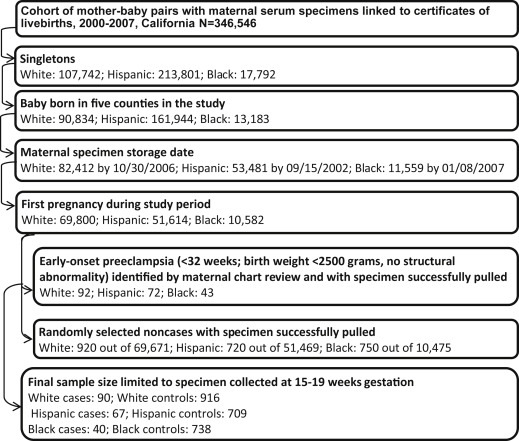
Control identification
Controls were noncases selected at an original sample size of ≥10 times the number of cases before specimen pulling using simple random sampling within the same race-ethnicity-specific singleton live-birth cohort as cases. Births with adverse outcomes such as late-onset preeclampsia, preterm birth, and low birthweight were also eligible controls. Controls of white, Hispanic, and black origin were drawn separately for each racial-ethnic group. For women with multiple pregnancies during the study period, only the first pregnancy was chosen. Control births without banked serum specimens or with structural malformations according to the California Birth Defects Monitoring Program registry were excluded. The final sample included 916 white, 709 Hispanic, and 738 black controls ( Figure ). Gestational age estimates for controls were based on ultrasound (55.8%), date of last menstrual period (34.9%), and physical exam (9.3%) from the California Prenatal Screening Program.
Biological specimens
Banked maternal blood specimens were retrieved from the California Biobank Program. As part of the state prenatal screening program, obstetrical care service providers at doctors’ offices or drawing stations collected venous blood from pregnant women who voluntarily enrolled at 15-20 weeks’ gestation. The blood, which was collected in serum separator tubes, was sent to 1 of the 7 newborn and prenatal screening laboratories by mail under ambient conditions. Specimens arrived for triple-marker prenatal testing within 7 days of collection (median time 3 days). After routine testing, leftover specimens were refrigerated and then processed for long-term storage at –20°C. Women consented to the routine prenatal screening test in writing and were notified that the blood becomes the property of the state, which allows for a variety of approved research uses of the leftover blood under the highest level of security and confidentiality standards. While women have the option to request the specimen not be used for research purposes, very few assert this right. Specimen vials from cases and controls were intermingled, with vial positions randomly assigned during shipping and laboratory testing. Laboratory personnel were unaware of subjects’ case-control status.
Laboratory methods
Maternal serum levels of PlGF, sEng, and sVEGFR-1 were determined by immunoassays (R&D Systems Inc, Minneapolis, MN) utilizing the quantitative sandwich enzyme immunoassay technique. Laboratory testing was conducted at the research core laboratory of Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. Standard serum specimens were used in each laboratory run and analyte value was determined by interpolation from standard curves. The assays utilized 120, 25, and 25 μL of serum for the PlGF, sEng, and VEGFR-1 assays, respectively. The interassay and intraassay coefficients of variation obtained in the laboratory were 4.6% and 3.6% for PlGF, 5.3% and 4.3% for sEng, and 2.8% and 2.7% for sVEGFR-1, respectively. The sensitivity of the assays was 5.7 pg/mL for PlGF, 40.0 pg/mL for sEng, and 12.9 pg/mL for sVEGFR-1.
Data analysis
The laboratory levels of PlGF, sEng, and sVEGFR-1 were linked to birth records, prenatal screening program data, and maternal hospital chart data (cases only) for analysis. Maternal characteristics were compared between cases and controls. Gestational weeks at delivery and at preeclampsia diagnosis for cases were compared among the 3 racial-ethnic groups. The χ 2 test and Student t test were used to estimate the statistical significance of the comparisons for categorical and continuous variables, respectively. Natural logarithmic transformed circulating levels of PlGF, sEng, and sVEGFR-1 were normally distributed in cases and controls. Means (log pg/mL) and SE were calculated for cases and controls of each racial-ethnic group and were adjusted by maternal age, education, parity, specimen storage year, gestational age at specimen collection, and maternal weight at or near specimen collection. The adjusted logarithmic means were compared using Tukey honestly significant difference test between cases and controls in each racial-ethnic group and between racial-ethnic groups among cases and controls, respectively. Odds ratios (OR) and 95% confidence intervals (CI) derived from binomial logistic regressions were used to measure effect magnitude of analyte levels in association with case-control status. The logarithmic transformed levels (log pg/mL) of the 3 analytes were used in the logistic regression to reduce both variance and outlier influence. Race-ethnicity is a significant effect modifier for PlGF and sEng in association with early-onset preeclampsia and therefore association results were presented separately for each race-ethnicity group. Maternal age, education, parity, specimen storage year, gestational weeks at specimen collection, and maternal weight at specimen collection were included as potential confounders. Standard Z test was conducted to compare OR between racial-ethnic groups. Given the above-mentioned final sample sizes, this study had adequate power of ≥80% to detect association of moderate or higher effect estimates (OR ≥2.6) between cases and controls in each racial-ethnic group under a logistic model with a 2-sided test.
All of the statistical analyses were conducted using software (SAS, Version 9.2 for Windows; SAS Institute, Cary, NC). The study protocol was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects (project no. 10-10-52).
Materials and Methods
Case identification
All early-onset preeclampsia cases (n = 197) evaluated in this study were identified by the California Very Preterm Birth Study from prenatal screening records and validated via chart review at the hospital of delivery. Detailed study design and methods have been published elsewhere. Briefly, very preterm births of white, Hispanic, or black origin were identified from a population-based cohort created through record linkage of 346,456 mother-baby pairs with stored maternal serum specimens linked to certificates of live births in California from January 2000 through April 2007. White and Hispanic births were defined as both parents being non-Hispanic white and Mexican Hispanic white, respectively, based on birth records. Mexican Hispanic white referred to self-reported white with Hispanic ethnicity origin of Mexican, Mexican-American, or Chicano. Black births were defined as black mothers of any ethnicity, regardless of father’s race-ethnicity. All of these women participated in routine prenatal screening for aneuploidies and neural tube defects between 15-20 weeks through the Genetic Disease Screening Program within the California Department of Public Health and had serum banked after screening. Study samples were limited to singleton live births delivered in 5 southern California counties (Los Angeles, Orange, Riverside, San Bernardino, and San Diego). Within this large cohort, the medical records of 1117 chronologically identified very preterm births of <32 weeks gestation (429 whites, 440 Hispanics, and 248 blacks) were abstracted to distinguish very preterm births with known causes including preeclampsia, which formed the case group for this study. Cases with a birthweight >2500 g were excluded based on suspected misdating. Pregnancies with structural abnormalities reported on delivery records or identified by the California Birth Defects Monitoring Program registry were also excluded.
Preeclampsia was defined as hypertension accompanied by proteinuria with onset >20 weeks’ gestation in the California Very Preterm Birth Study. Hypertension was defined as blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic on 2 occasions 6 hours apart. Proteinuria was defined as ≥0.3 g of protein in a 24-hour urine collection or ≥1+ dipstick on a urine test on 2 occasions 6 hours apart. Early-onset preeclampsia was defined as preeclampsia with delivery <32 weeks’ gestation. Gestational age estimates for cases were mostly based on ultrasound (61.5%) and date of last menstrual period (29.5%) from the California Prenatal Screening Program with a small part (9.0%) based on maternal hospital chart review.
Hospital chart review identified 207 cases of early-onset preeclampsia with a maternal midtrimester specimen available for laboratory testing. Ten of the 207 cases were excluded due to maternal specimens collected beyond the gestational window of 15-20 weeks. The final sample included 90 white, 67 Hispanic, and 40 black cases. Details of the sample selection are listed in the Figure .
Control identification
Controls were noncases selected at an original sample size of ≥10 times the number of cases before specimen pulling using simple random sampling within the same race-ethnicity-specific singleton live-birth cohort as cases. Births with adverse outcomes such as late-onset preeclampsia, preterm birth, and low birthweight were also eligible controls. Controls of white, Hispanic, and black origin were drawn separately for each racial-ethnic group. For women with multiple pregnancies during the study period, only the first pregnancy was chosen. Control births without banked serum specimens or with structural malformations according to the California Birth Defects Monitoring Program registry were excluded. The final sample included 916 white, 709 Hispanic, and 738 black controls ( Figure ). Gestational age estimates for controls were based on ultrasound (55.8%), date of last menstrual period (34.9%), and physical exam (9.3%) from the California Prenatal Screening Program.
Biological specimens
Banked maternal blood specimens were retrieved from the California Biobank Program. As part of the state prenatal screening program, obstetrical care service providers at doctors’ offices or drawing stations collected venous blood from pregnant women who voluntarily enrolled at 15-20 weeks’ gestation. The blood, which was collected in serum separator tubes, was sent to 1 of the 7 newborn and prenatal screening laboratories by mail under ambient conditions. Specimens arrived for triple-marker prenatal testing within 7 days of collection (median time 3 days). After routine testing, leftover specimens were refrigerated and then processed for long-term storage at –20°C. Women consented to the routine prenatal screening test in writing and were notified that the blood becomes the property of the state, which allows for a variety of approved research uses of the leftover blood under the highest level of security and confidentiality standards. While women have the option to request the specimen not be used for research purposes, very few assert this right. Specimen vials from cases and controls were intermingled, with vial positions randomly assigned during shipping and laboratory testing. Laboratory personnel were unaware of subjects’ case-control status.
Laboratory methods
Maternal serum levels of PlGF, sEng, and sVEGFR-1 were determined by immunoassays (R&D Systems Inc, Minneapolis, MN) utilizing the quantitative sandwich enzyme immunoassay technique. Laboratory testing was conducted at the research core laboratory of Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. Standard serum specimens were used in each laboratory run and analyte value was determined by interpolation from standard curves. The assays utilized 120, 25, and 25 μL of serum for the PlGF, sEng, and VEGFR-1 assays, respectively. The interassay and intraassay coefficients of variation obtained in the laboratory were 4.6% and 3.6% for PlGF, 5.3% and 4.3% for sEng, and 2.8% and 2.7% for sVEGFR-1, respectively. The sensitivity of the assays was 5.7 pg/mL for PlGF, 40.0 pg/mL for sEng, and 12.9 pg/mL for sVEGFR-1.
Data analysis
The laboratory levels of PlGF, sEng, and sVEGFR-1 were linked to birth records, prenatal screening program data, and maternal hospital chart data (cases only) for analysis. Maternal characteristics were compared between cases and controls. Gestational weeks at delivery and at preeclampsia diagnosis for cases were compared among the 3 racial-ethnic groups. The χ 2 test and Student t test were used to estimate the statistical significance of the comparisons for categorical and continuous variables, respectively. Natural logarithmic transformed circulating levels of PlGF, sEng, and sVEGFR-1 were normally distributed in cases and controls. Means (log pg/mL) and SE were calculated for cases and controls of each racial-ethnic group and were adjusted by maternal age, education, parity, specimen storage year, gestational age at specimen collection, and maternal weight at or near specimen collection. The adjusted logarithmic means were compared using Tukey honestly significant difference test between cases and controls in each racial-ethnic group and between racial-ethnic groups among cases and controls, respectively. Odds ratios (OR) and 95% confidence intervals (CI) derived from binomial logistic regressions were used to measure effect magnitude of analyte levels in association with case-control status. The logarithmic transformed levels (log pg/mL) of the 3 analytes were used in the logistic regression to reduce both variance and outlier influence. Race-ethnicity is a significant effect modifier for PlGF and sEng in association with early-onset preeclampsia and therefore association results were presented separately for each race-ethnicity group. Maternal age, education, parity, specimen storage year, gestational weeks at specimen collection, and maternal weight at specimen collection were included as potential confounders. Standard Z test was conducted to compare OR between racial-ethnic groups. Given the above-mentioned final sample sizes, this study had adequate power of ≥80% to detect association of moderate or higher effect estimates (OR ≥2.6) between cases and controls in each racial-ethnic group under a logistic model with a 2-sided test.
All of the statistical analyses were conducted using software (SAS, Version 9.2 for Windows; SAS Institute, Cary, NC). The study protocol was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects (project no. 10-10-52).
Results
The prevalence of early-onset preeclampsia (<32 weeks’ gestation) was 0.13% for whites (92 of 69,800), 0.14% for Hispanics (72 of 51,614), and 0.41% for blacks (43 of 10,582) based on the linked birth cohort for this study ( Figure ), within the lower range previously reported. Compared to controls, white cases were more likely to be nulliparous (81.11% vs 50.11%, P < .05), and Hispanic cases were older than controls (28.61 years on average vs 26.22 years, P < .05), as presented in Table 1 . Cases of white and Hispanic origin were heavier at or near the time of specimen collection than controls (76.20 kg on average vs 70.02 kg in whites and 76.33 kg vs 67.22 kg in Hispanics, P < .05, respectively). Maternal education and gestational age at specimen collection were not associated with case-control status in any racial-ethnic group. Gestational age at delivery and at preeclampsia diagnosis among cases did not differ across racial-ethnic groups.



