Chapter 321 Pyloric Stenosis and Other Congenital Anomalies of the Stomach
321.1 Hypertrophic Pyloric Stenosis
Clinical Manifestations
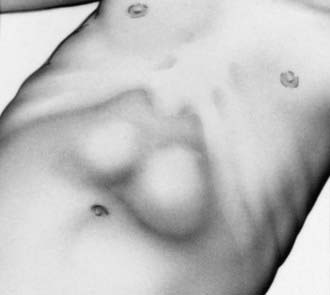
The diagnosis has traditionally been established by palpating the pyloric mass. The mass is firm, movable, ∼2 cm in length, olive shaped, hard, best palpated from the left side, and located above and to the right of the umbilicus in the mid-epigastrium beneath the liver’s edge. The olive is easiest palpated after an episode of vomiting. After feeding, there may be a visible gastric peristaltic wave that progresses across the abdomen (Fig. 321-1).
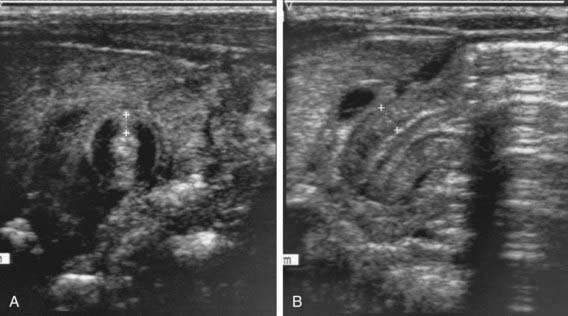
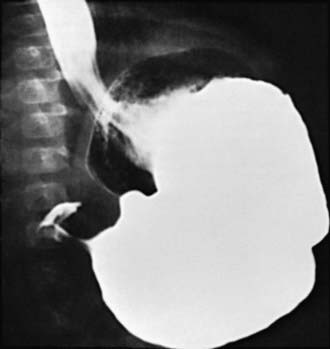
Two imaging studies are commonly used to establish the diagnosis. Ultrasound examination confirms the diagnosis in the majority of cases. Criteria for diagnosis include pyloric thickness 3-4 mm, an overall pyloric length 15-19 mm, and pyloric diameter of 10-14 mm (Fig. 321-2). Ultrasonography has a sensitivity of ∼95%. When contrast studies are performed, they demonstrate an elongated pyloric channel (string sign), a bulge of the pyloric muscle into the antrum (shoulder sign), and parallel streaks of barium seen in the narrowed channel, producing a “double tract sign” (Fig. 321-3).
Differential Diagnosis
Gastric waves are occasionally visible in small, emaciated infants who do not have pyloric stenosis. Infrequently, gastroesophageal reflux, with or without a hiatal hernia, may be confused with pyloric stenosis. Gastroesophageal reflux disease can be differentiated from pyloric stenosis by radiographic studies. Adrenal insufficiency from the adrenogenital syndrome can simulate pyloric stenosis, but the absence of a metabolic acidosis and elevated serum potassium and urinary sodium concentrations of adrenal insufficiency aid in differentiation (Chapter 570
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree