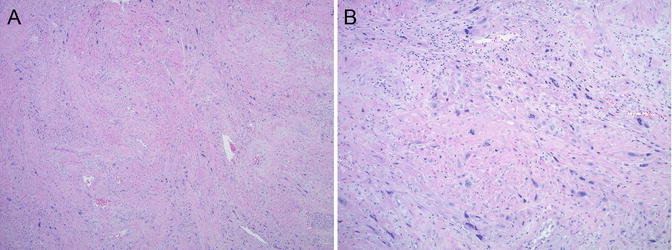
Fig. 5.1
Microscopic features of complete hydatidiform mole (CHM). (a, b) Well-developed CHM with large hydropic villi and central cistern formation. Circumferential trophoblast hyperplasia is also present. (c, d) Early CHM with polypoid, “cauliflower-like” villi; hypercellular, myxoid villous stroma with karyorrhexis; and circumferential trophoblast hyperplasia
Partial Hydatidiform Mole
The specimen volume in partial moles is typically less than that of CHM, and grossly visible hydropic change is rare, especially during the first and early second trimester. Fetal development may be seen, usually with mild to moderate symmetrical intrauterine growth restriction and characteristic malformations (e.g., syndactyly) [41].
Histologically, there are usually two populations of chorionic villi in PHM—large, hydropic villi (ranging between 1 and 6 mm in size) in the background of small or normal appearing ones. The villous contour is irregular, scalloped with surface invaginations, and round to oval trophoblastic pseudo-inclusions. Trophoblastic hyperplasia is typically mild to moderate and focal, without significant cytological atypia. Cistern formation is not uncommon. Fetal vessels with nucleated red blood cells are often seen (Fig. 5.2).
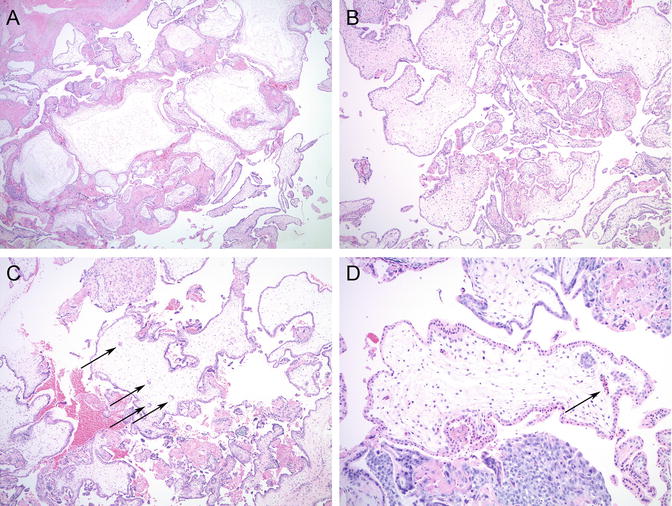

Fig. 5.2
Microscopic features of partial hydatidiform mole (PHM). (a, b) Two villous populations: large hydropic villi with occasional cistern formation in the background of smaller villi. (c) Irregular villous contours and trophoblastic pseudo-inclusions (arrows). (d) Fetal vessels with nucleated red blood cells are commonly seen (arrow)
Ancillary Studies
The diagnosis of hydatidiform moles is often challenging based on clinical and morphological features alone. The microscopic changes are not entirely specific and show significant overlap between complete and partial mole, especially when evacuated at an early gestational age. In addition, non-molar gestations with or without identifiable genetic abnormalities can also mimic hydatidiform moles at the morphologic level. Ancillary studies are often necessary to differentiate between complete and partial hydatidiform moles and to rule out non-molar mimics.
Ploidy Analysis
Determination of the number of complete haploid sets of chromosomes can separate diploid gestations from triploid, tetraploid, or other aneuploid ones. However, it does not provide information about the parental origin of chromosome sets; thus, a diploid CHM cannot be separated from diploid non-molar hydropic abortion based on DNA ploidy. In addition, it is unable to differentiate between triploid—diandric monogynic—partial moles and non-molar digynic monoandric triploidy, which constitute at least one third of all triploid gestations and are not associated with increased risk of GTN [29, 42, 43]. Conventional karyotyping has been used for several decades to assess ploidy and is very helpful in identifying chromosomal trisomy syndromes, which often mimic PHM morphologically. However, it requires fresh tissue and is time and labor intensive, limiting its utility in routine practice. Ploidy analysis can also be performed on formalin-fixed paraffin-embedded material by flow cytometry, although it may be prone to technical problems and interpretation errors, leading to potential misclassification of ploidy [44–47]. Another technique—polymorphic deletion probe (PDP) fluorescent in situ hybridization (FISH) —has been recently reported for chromosomal enumeration in suspected molar gestations, but similar to the other methods, it suffers from both technical limitations and interpretation problems [48].
P57 Immunohistochemistry
Various immunohistochemical markers, including cell cycle proteins (E2F-1, CDK2, cyclin E, p27, p57) and proliferation markers (proliferation cell nuclear antigen [PCNA], Ki-67), have been explored for their diagnostic utility in hydatidiform moles [49–51]. However, only p57 immunostain has been found useful in routine diagnostic pathology practice. P57 gene is located on chromosome 11p15.5 and encodes a cyclin-dependent kinase inhibitor protein [52, 53]. Since the gene is paternally imprinted—preferentially expressed from the maternal allele—maternal genetic material is necessary for a normal p57 protein expression pattern: strong nuclear staining in cytotrophoblasts, intermediate trophoblasts, intervillous trophoblast islands, and villous stromal cells and absent staining in syncytiotrophoblasts [54]. Normal placentas, non-molar hydropic abortions, chromosomal trisomies, digynic triploid cases, and partial moles all show normal p57 staining patterns, due to the presence of maternal genetic material in their genomes. Complete moles, on the other hand, lack p57 immunoreactivity in cytotrophoblast and villous stromal cells, but retain p57 expression in intervillous intermediate trophoblasts and villous endothelial cells (Fig. 5.3). Maternal decidua always shows positive nuclear staining, serving as an internal positive control.
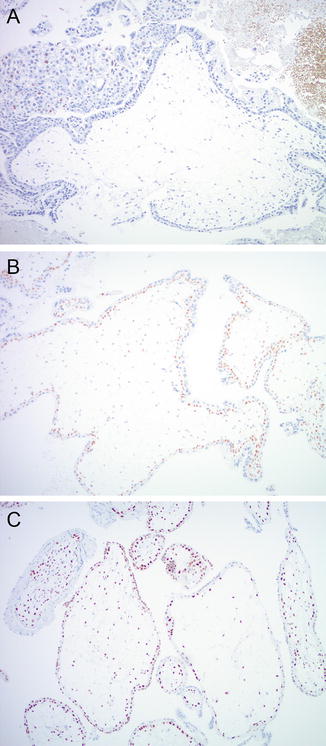

Fig. 5.3
P57 immuno histochemistry. (a) CHM with absent p57 staining in villous stroma and cytotrophoblast. Intervillous intermediate trophoblast shows positive nuclear staining (upper left corner). PHM (b) and non-molar hydropic abortion (c) show normal p57 immunostaining pattern
P57 immunohistochemistry is a useful adjunct test in the diagnostic workup of hydatidiform moles, as it can separate CHM from PHM and from other non-molar hydropic gestations. However, partial moles and their non-molar mimics contain maternal genetic material and thus show normal p57 staining pattern and cannot be separated from each other based on p57 immunohistochemistry. Additionally, some complete moles may show focal p57 staining, due to incomplete imprinting, twin gestation (admixture of CHM and normal villi), or rare androgenetic/biparental mosaic/chimeric gestation [55, 56]. Another rare potential pitfall is CHM with retention of maternal chromosome 11, resulting in normal p57 expression [57, 58], and rare p57-negative PHM due to loss of maternal chromosome 11 [59]. The p57 immunohistochemical pattern of biparental complete moles is identical to androgenetic CHM as a result of mutations of the NLRP7 or KHDC3L genes and disruption of the normal imprinting pattern [60].
Short Tandem Repeat Genotyping
Short tandem repeats (STR) are repetitive, genetically stable DNA sequences of 2–7 nucleotides, which are highly prevalent in the noncoding regions of the genome [61]. STR polymorphism —difference in the number of repeats at each STR locus among different members of a species—can be analyzed and used to distinguish between individuals. STR genotyping is widely used for identity testing in forensics and more recently has also become part of the routine diagnostic workup for molar gestations at some large academic centers [21, 62, 63].
One of the advantages of STR genotyping compared to other ancillary molecular techniques is that it can be performed on formalin-fixed paraffin-embedded tissue samples, following dissection of pure maternal and fetal tissues from unstained sections. Numerous commercial kits , e.g., PowerPlex® 16 System (Promega) are available for DNA extraction and PCR amplification. Comparison of the allelic profiles between maternal and fetal (chorionic villous) tissues at 15 STR loci provides information about the parental genetic contribution to the villous tissue and the relative proportions of maternal and paternal genetic material. Complete moles show paternal-only alleles—either in a homozygous or heterozygous pattern —in at least two informative STR loci (Fig. 5.4). PHM can be diagnosed in the presence of two unique paternal alleles in addition to one maternal allele in at least two loci (dispermic or heterozygous PHM) or one paternal allele in duplicate quantity and one maternal allele at every STR locus (monospermic or homozygous PHM) (Fig. 5.5). Digynic triploidy can be reliably distinguished from PHM using STR genotyping by the presence of one paternal and two maternal alleles. A biallelic profile with balanced maternal and paternal contributions indicates a non-molar abortion (Fig. 5.6). Chromosomal trisomies may also be identified by genotyping as a single allelic gain, although not all chromosomes are represented among the 15 STR loci (Fig. 5.7) [64]. Rare potential pitfalls of genotyping interpretation also exist and require close morphologic correlation and p57 immunostaining in some cases to avoid misclassification. For example, biparental CHM shows a biparental allelic profile on genotyping; however, the histological features and p57 expression pattern are diagnostic and are indistinguishable from those of a diandric complete mole [23, 25]. A case of egg donor pregnancy has also been reported with morphologic features suspicious for PHM and genotyping results mimicking a complete mole, due to lack of maternal alleles in the villous tissue [65]. In addition, twin pregnancy with coexisting CHM and normal fetus and cases with mosaicism/chimerism could also interfere with the interpretation of genotyping data [66, 67].
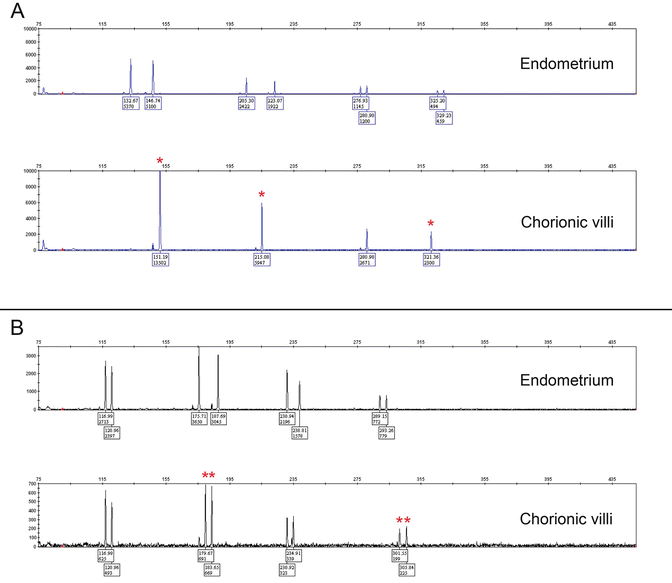
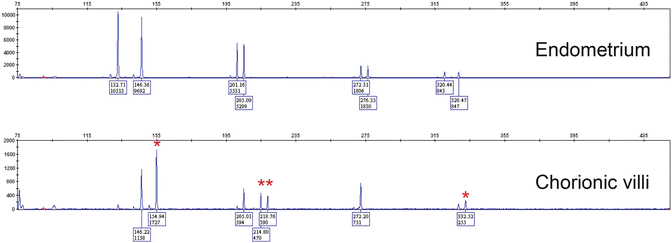
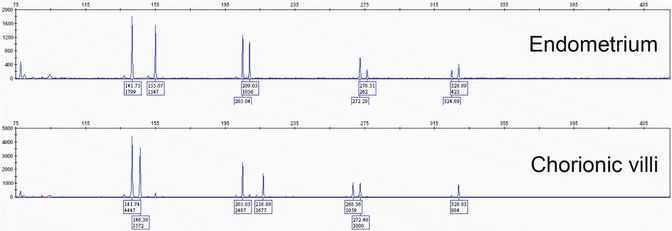
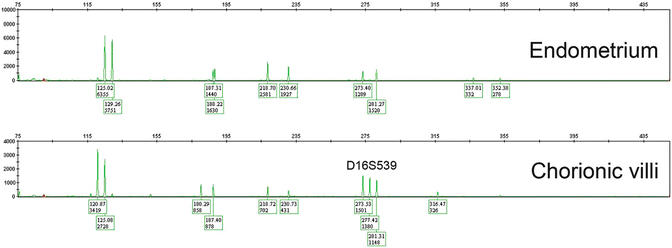

Fig. 5.4
STR genotyping of complete hydatidiform moles (CHM ). (a) In homozygous (monospermic) CHM, the chorionic villi show a unique paternal allele (asterisk) in duplicate quantity and absence of maternal alleles in at least two informative loci. (b) In heterozygous (dispermic) CHM, there are two unique paternal alleles (asterisk) and absence of maternal alleles in at least two informative loci

Fig. 5.5
STR genotyping of partial hydatidiform mole (PHM). Dispermic PHM shows a triploid pattern with two unique paternal alleles (double asterisk) or one paternal allele (asterisk) in duplicate quantity in addition to one maternal allele

Fig. 5.6
Non-molar hydropic abortion showing a balanced biparental allelic pattern on genotyping

Fig. 5.7
Trisomy 16 . Three alleles identified at locus D16S539, other loci show normal biparental allelic pattern
Differential Diagnosis
Spontaneous non-molar hydropic abortions may show significant villous enlargement and edema, occasionally even with cistern formation, mimicking a complete or partial mole on the morphologic level. However, the villous contour is round or oval without invaginations, and trophoblastic hyperplasia is absent or mild and polarized (not circumferential) (Fig. 5.8). They show normal p57 expression pattern and a balanced biallelic profile on genotyping.
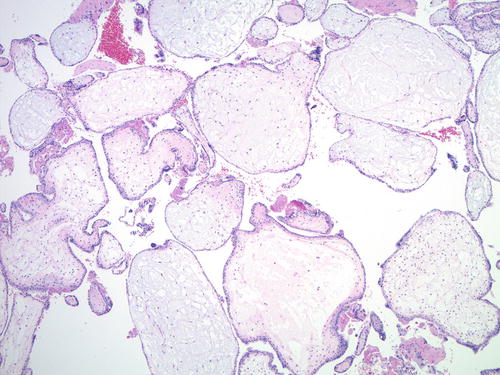

Fig. 5.8
Non-molar hydropic abortion . The villous shape is round or oval; no trophoblastic pseudo-inclusions or significant trophoblast hyperplasia is seen
The morphologic changes of an early non-molar gestation and a very early complete mole (VECM) may overlap in the form of villous size and villous stromal cellularity. Trophoblastic proliferation —even if it is only mild or moderate—is circumferential or random in VECM, in contrast to the polarized trophoblastic proliferation of early normal pregnancy. Presence of fetal parts and well-formed villous stromal vessels with nucleated red blood cells essentially rules out a complete mole. P57 immunohistochemistry and genotyping can be used to resolve difficult cases.
Chromosomal trisomies (especially trisomies 7, 8, 13, 15, 16, 18, 21, and 22) often have hydropic, irregularly shaped chorionic villi with frequent trophoblastic pseudo-inclusions, morphologically simulating PHM (Fig. 5.9). Trophoblastic hyperplasia may also be present, more commonly in trisomies involving chromosomes 7, 15, 21, and 22 [68]. However, when compared with trisomy syndromes and non-molar hydropic abortions, the combination of cistern formation and maximum villous size of ≥2.5 mm has been shown to have a 90 % positive predictive value for partial mole [64]. P57 immunohistochemistry does not help distinguishing PHM from trisomies, as they both show normal expression pattern due to the presence of maternal genetic material. Genotyping can identify diandric triploidy—diagnostic of partial mole—and separate it from common trisomy syndromes showing a single allelic gain.
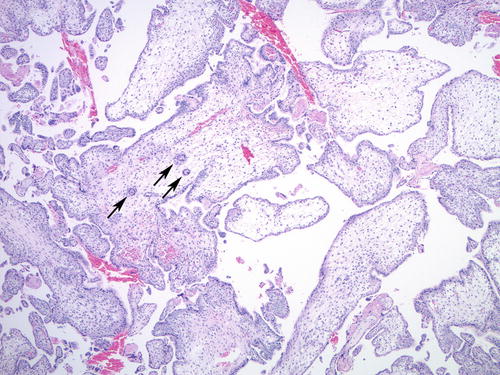

Fig. 5.9
Trisomy 13 with villous hydrops and irregular villous contours, resulting in trophoblastic pseudo-inclusions (arrows)
Digynic monoandric triploidy —comprising approximately one third of all triploid gestations—may also present a diagnostic challenge morphologically and also on ploidy analysis. Similar to PHM, it may show villous contour irregularity with trophoblastic pseudo-inclusions, mild hydropic change, and syncytiotrophoblast sprouts (Fig. 5.10) [69]. However, unlike PHM, digynic triploidy is not associated with increased risk of persistent GTD or GTN. Molecular genotyping can be used to determine the parental origin of the haploid chromosome sets in the triploid genome and precisely separate the two entities.
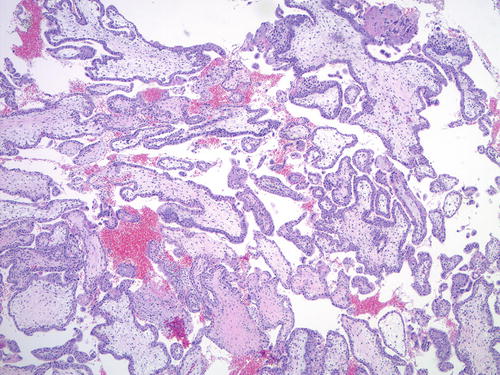

Fig. 5.10
Digynic triploidy showing mild villous hydrops and scalloped villous surface, mimicking PHM
Placental mesenchymal dysplasia is a late gestational age mimic of PHM, presenting with stem and terminal villous hydrops, rare cistern formation, aneurysmal stem vessels, and peripheral stem villous chorioangiomatoid change, usually in late second trimester [43, 70]. Trophoblastic hyperplasia and trophoblastic pseudo-inclusions are typically absent [70]. Fetal abnormalities are common, in the form of intrauterine growth restriction or Beckwith-Wiedemann syndrome [71]. Genotyping shows a balanced biparental allelic pattern, allowing clear separation from a partial molar gestation.
Proposed Diagnostic Algorithm of Hydatidiform Moles
Integration of ancillary techniques into the routine diagnostic workup of molar gestations is necessary to avoid diagnostic misclassification based solely on morphologic findings. Two algorithmic approaches have been recently proposed to combine morphologic evaluation with p57 immunohistochemistry and DNA genotyping [55, 72, 73]. According to one approach, all cases with morphologic suspicion for either complete or partial hydatidiform mole are subjected to p57 immunohistochemistry first, and cases with absent staining are diagnosed as CHM [55, 73]. Cases with equivocal staining pattern or morphological features are further analyzed by STR genotyping. Another algorithm advocates genotyping on all cases with morphologic features of complete or partial mole, to obtain a definitive diagnosis based on the parental genetic contribution to the villous tissue [72]. Rare cases with a biallelic genotyping pattern and strong morphologic suspicion for CHM are also evaluated by p57 immunohistochemistry as a second step, to rule out a biparental CHM.
The two algorithmic approaches may also be combined: cases with histologic features of CHM can be subjected to p57 immunostaining, and the lack of reactivity would confirm the diagnosis of complete mole. However, cases showing characteristics of PHM microscopically are evaluated by genotyping (without p57 immunostaining) [74].
Invasive and Metastatic Hydatidiform Mole
Invasive mole is characterized by myometrial and/or vascular invasion by molar villi and is seen in approximately 10–15 % of CHM and up to 5 % of PHM cases [75–79]. The molar villi may rarely spread to the broad ligament, vagina, and vulva and even metastasize to distant sites, such as lungs [80, 81]. Histologically, invasive CHM has diffuse villous hydrops and trophoblastic hyperplasia, which occasionally may be exuberant and shows marked cytological atypia, resembling choriocarcinoma in isolation (Fig. 5.11). Such lesions may be considered as “emerging” or “in situ” choriocarcinoma (see next section). Precise distinction between these entities is typically not crucial for clinical management purposes, as they are all encompassed under the term persistent GTD and are often treated based on clinical parameters alone [82].
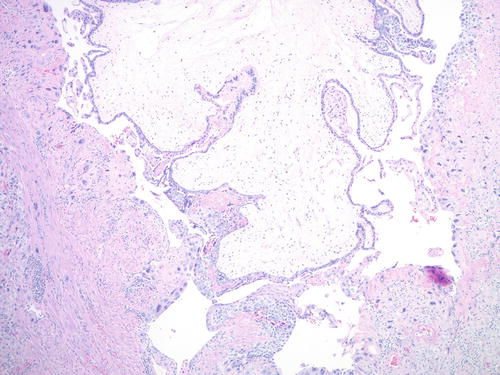

Fig. 5.11
Invasive comp lete mole. Large hydropic molar villi seen invading into the myometrium in a hysterectomy specimen
In Situ or Intraplacental Choriocarcinoma
Histological diagnostic criteria of choriocarcinoma traditionally include bilamellar growth pattern with mononuclear trophoblast rimmed by a layer of multinucleated syncytiotrophoblast, severe cytological atypia, high mitotic activity, and absence of chorionic villi. However, it has been proposed more recently that an intermediate or precursor lesion also exists: “emerging” or “in situ” choriocarcinoma in the presence of non-molar or molar villi with exuberant trophoblastic hyperplasia and marked cytological atypia (Fig. 5.12) [13, 83, 84]. It should also be noted that very early gestations may have foci of sheetlike trophoblastic proliferation with high mitotic activity mimicking an “in situ” choriocarcinoma, although significant cytologic atypia is absent.
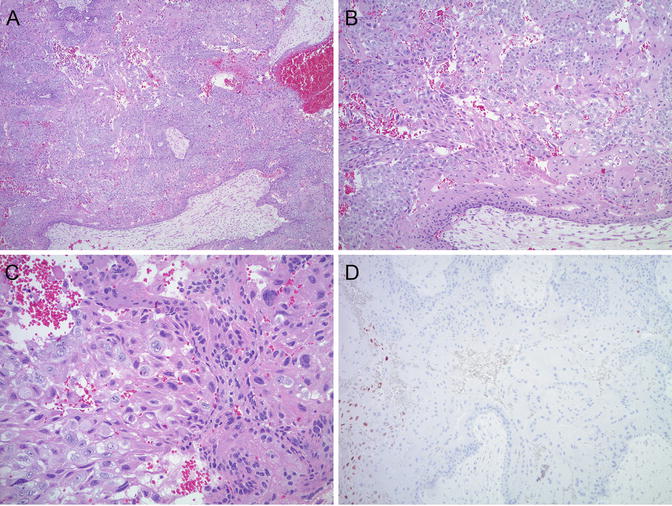

Fig. 5.12
(a–c) “Incipien t choriocarcinoma”: exuberant biphasic, atypical trophoblastic proliferation in the presence of complete molar villi. (d) P57 immunostain is negative in villous stroma and cytotrophoblast, confirming complete hydatidiform mole. Positive internal control in intermediate trophoblast (left side of image)
Rare cases of intraplacental choriocarcinoma have also been documented in a full-term placenta [8–12, 14, 15, 85]. The initial presentation of some of these patients was metastatic choriocarcinoma, and the intraplacental primary lesion was only discovered after careful reexamination of the placenta. As these lesions are often small—measuring less than 1 cm—they may be missed on gross examination. Hence, some investigators recommend sectioning of the placenta at 5 mm intervals and sampling of any hemorrhagic mass lesions [10].
Atypical Placental Site Nodule
Placental site nodule (PSN) or plaque—a benign, reactive proliferation of chorion laeve-type intermediate trophoblast —is most commonly an incidental finding in endometrial curettings, although patients may present with irregular uterine bleeding [86–89]. PSN is typically small, ranging from 4 to 10 mm in size [89]. Microscopically, it is characterized by haphazardly arranged mononuclear or less often multinucleated trophoblast in a hyalinized matrix, showing variable cellularity, often with zonation (Fig. 5.13). Mild nuclear atypia and nuclear pseudo-inclusions are not uncommon; however, mitotic figures are rare or absent. Immunohistochemical stains for cytokeratins (CAM 5.2, AE1/AE3, 34βE12), EMA, p63, human placental lactogen (hPL), and hCG show variable positivity [89]. The proliferative index by Ki-67 immunostain is less than 8 %, and cyclin E immun ostaining is weak or absent [87].
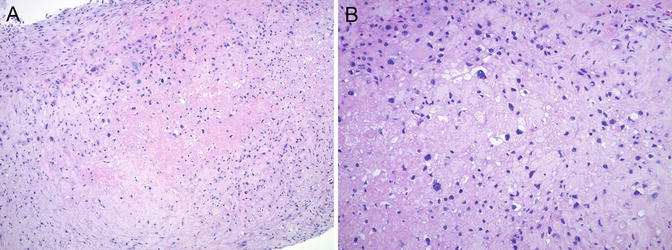

Fig. 5.13
Placental site nodule (PSN). (a) Small fragment of PSN in a cervical curettage specimen with relatively low cellularity and central hyalinization. (b) Mild nuclear atypia and multinucleation are not uncommon
Epithelioid trophoblastic tumor (ETT) on the other hand is a malignant neoplasm composed of chorion laeve-type intermediate trophoblast, usually forming a larger mass lesion (0.5–4 cm), often in the cervix or lower uterine segment [90, 91]. Unlike in PSN, moderate nuclear atypia and increased mitotic activity—typically ranging from 1 to 10/10 high power field (HPF)—are seen (Fig. 5.14). Rare cases with even higher mitotic count—48/10 HPF—have also been reported [91]. Central areas of eosinophilic, hyalinized material, and necrotic debris are also characteristic. Ki-67 immunostain shows >10 % positivity, and cyclin E immunostain is strongly, diffusely positive.
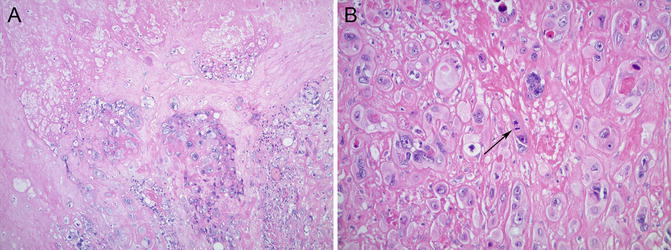

Fig. 5.14
Epithelioid trophoblastic tumor (ETT) shows increased cellularity, necrosis (a), and moderate to marked nuclear atypia with mitotic figures (arrow, b)
Atypical PSN (APSN) shows intermediate morphologic features between PSN and ETT—larger size, increased cellularity, moderate nuclear atypia, presence of mitotic figures, and Ki-67 proliferation index betw een 8 and 10 % [92]—and has been proposed as a precursor lesion to ETT [16, 93] (Fig. 5.15). Shih and Kurman reported intimate association between PSN and ETT within the same specimen in 2 of 14 ETT cases [90]. Transformation of PSN into ETT and/or PSTT has also been described in two recent case reports with both lesions present in the same specimen (curettage or hysterectomy) in close proximity, with a microscopic atypical transitional area in between [17, 94]. In a series of 42 PSNs, four cases showed atypical microscopic features, but no recurrences or GTN was seen on follow-up [87]. Most recently, 21 atypical PSNs—the largest series to date—have been reported, 3 of which (14 %) were associated with malignant GTD: one patient had concurrent atypical PSN and PSTT, one patient developed PSTT after 16 months, and one patient was diagnosed with ETT 6 months after the initial diagnosis [95]. Based on these data, it has been suggested that patients with APSN should be evaluated by imaging studies to rule out an underlying mass lesion and would require clinical follow-up due to the approximately 10–15 % risk of malignant GTD. Serum hCG measurement has also been recommended, although it appears to be unreliable for early detection of malignant transformation in this setting [95].
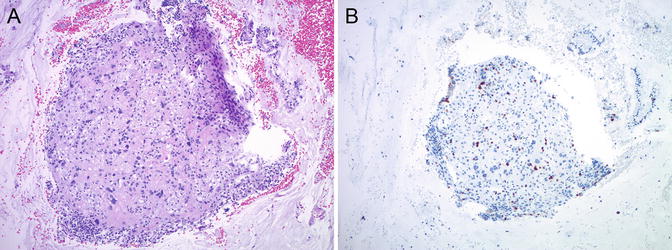

Fig. 5.15
Atypical place ntal site nodule (APSN). Small fragment (~2 mm) of APSN in a curettage specimen with high cellularity and at least moderate nuclear atypia (a). Ki-67 immunostain shows a slightly increased proliferation index (b)
Atypical Exaggerated Placental
Exaggerated placental site (EPS) is a reactive proliferation of intermediate trophoblast at the implantation site in a concurrent—or recent—normal, ectopic, or molar pregnancy. The trophoblastic cells are large, pleomorphic with abundant eosinophilic cytoplasm, and infiltrate the underlying myometrium dissecting between individual smooth muscle fibers. Most lesional cells are mononuclear, but variable number of multinucleated trophoblasts are also present which are evenly distributed throughout the lesion (Fig. 5.16). Mitotic figures are absent, and the Ki-67 proliferation index is low (less than 1 %). Chorionic villi are often seen adjacent to EPS, which likely represents the upper end of the morphological spectrum of normal implantation site.