John T. Sullivan, MD, MBA
CATEGORIZATION OF PULMONARY EDEMA
PHYSIOLOGY OF PREGNANCY AND PULMONARY EDEMA
• Pregnancy-Associated Cardiac Disease
EVALUATION OF THE PATIENT WITH SUSPECTED PULMONARY EDEMA
• History and Physical Examination
INTRODUCTION
Pulmonary edema is defined as the abnormal accumulation of fluid in the interstitial and alveolar spaces of the lung. This accumulation can ultimately impair gas exchange by leading to decreased diffusion of carbon dioxide and oxygen in the alveoli. The resulting impact on oxygenation and ventilation carries significant morbidity and has the potential to be life threatening if severe.1 It is important to note that pulmonary edema is a clinical symptom that represents the manifestations of several distinct pathological processes. In the setting of pregnancy, pulmonary edema is relatively rare, but it can present in patients in the antepartum, intrapartum, and postpartum periods.2 Maternal pulmonary edema is particularly concerning as the parturient already has decreased pulmonary functional reserve because of the physiologic changes associated with pregnancy, increased metabolic needs, and the gestating fetus is less able to tolerate a hypoxic maternal environment. Furthermore, several coexisting maternal conditions and complications, such as underlying cardiac disease, preeclampsia, sepsis, multiple gestations, as well as routinely used treatments (eg, tocolysis) can lead to the development of pulmonary edema or worsen the severity of the condition.2,3
INCIDENCE
Pulmonary edema occurring during pregnancy or in the postpartum period has a reported incidence of 0.08%.2 Despite the low incidence of the disease, it is associated with a disproportionate amount of morbidity and mortality.4 The period of pregnancy at greatest risk for presentation appears to be the immediate postpartum. In the largest case series available from a high-volume tertiary care hospital (51 cases over 10 years and 62,917 pregnancies), 39% of cases presented in the first 24 hours following delivery. This compares with 47% of cases diagnosed in the entire antepartum period (mean gestational age of at presentation approximately 31 weeks). Only 14% of patients in this case series developed pulmonary edema during labor. This distribution of the timing for presentation of pulmonary edema is consistent with previous reports.2,5
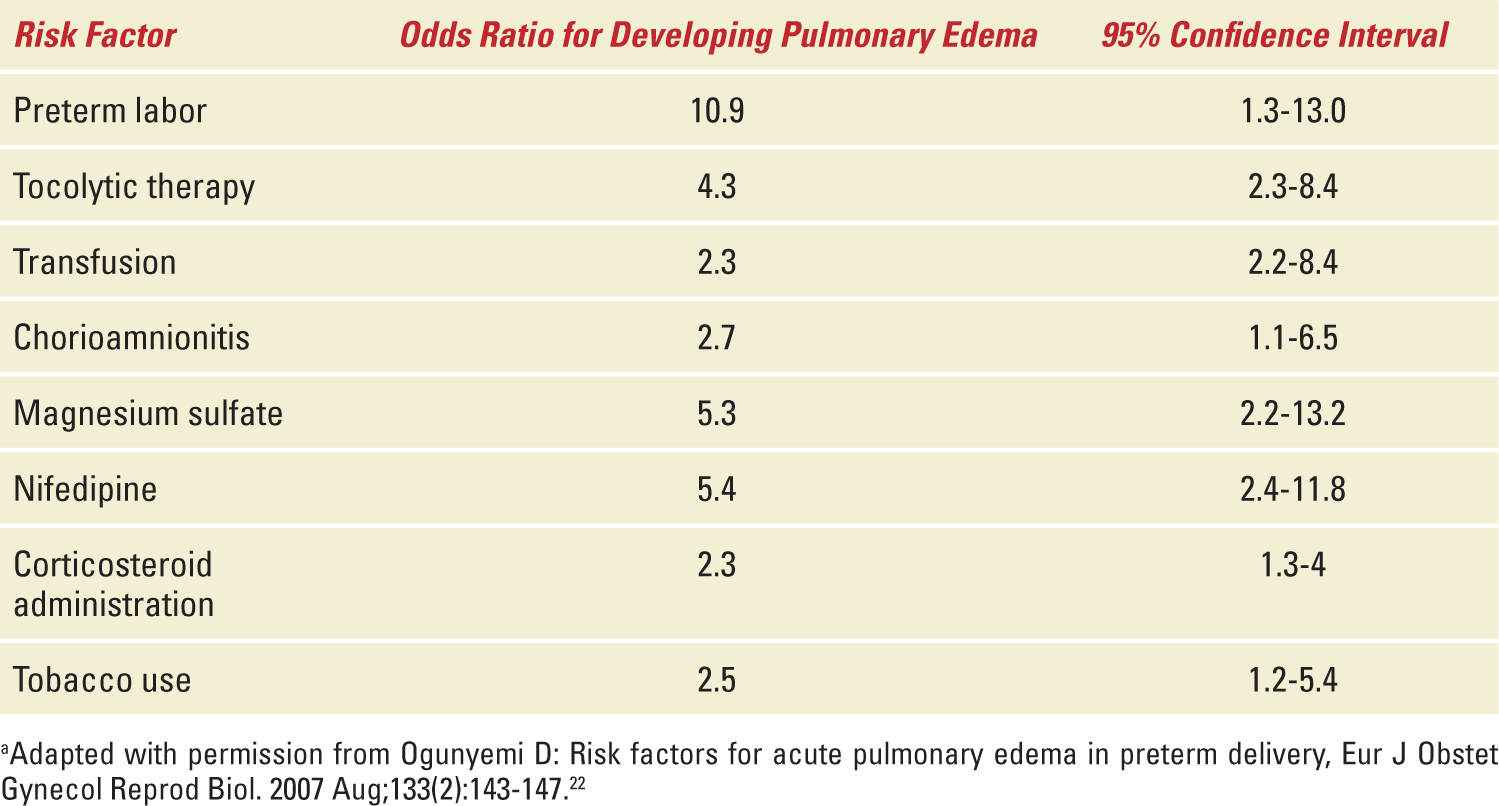
Several predisposing factors have been identified in women who develop pulmonary edema during pregnancy. The most common attributable causes are pre-existing cardiac disease (25.5% of all cases), tocolytic use (25.5%), iatrogenic fluid overload (21.5%), and preeclampsia (18%).2 Patients with these risk factors should significantly raise clinical suspicion for pulmonary edema in the setting of new-onset respiratory compromise. Other less common risk factors for the development of pulmonary edema have been identified in some but not all retrospective analyses (Table 8-1).
TABLE 8-1 | Common Risk Factors for Pulmonary Edema in Preterm Deliverya |
CATEGORIZATION OF PULMONARY EDEMA
Two distinct and fundamentally different types of pulmonary edema have been broadly categorized: cardiogenic pulmonary edema (also referred to as hydrostatic or hemodynamic pulmonary edema) and noncardiogenic pulmonary edema (may include increased permeability pulmonary edema, acute lung injury, or acute respiratory distress syndrome (ARDS). The clinical manifestation of both types involve the accumulation of fluid in the extravascular portions of the lung, but the mechanisms behind this accumulation are drastically different.6
Cardiogenic pulmonary edema results from elevated intravascular pressures that create a hydrostatic gradient from the pulmonary vascular endothelial beds to the interstitial and alveolar spaces of the lungs. The hydrostatic pressure gradient is generated through excessively high left ventricular end-diastolic pressure, which can result from underlying cardiac functional problems (ie, systolic or diastolic heart failure), valvular disease, or volume overload. Acute elevations of hydrostatic pressure overwhelm the lymphatic system resulting in alveolar fluid accumulation, increase in dead space, and diminished alveolar gas exchange. Although this pressure gradient causes extravasation of fluid into interstitial and alveolar spaces, the underlying lung microvascular integrity is maintained.6
The mechanism behind the development of noncardiogenic pulmonary edema is a pathologic increase in the vascular permeability of the lung, which allows for accumulation of fluid and proteins into the alveoli that detrimentally affects the lung’s ability to participate in gas exchange. Protein in the interstitial space and airspaces worsens the edema as it disrupts the normal oncotic pressure gradient that favors fluid shifts back to the intravascular space. The increased presence of protein in alveolar fluid is a key distinguishing factor between the edema generated by cardiogenic and noncardiogenic mechanisms. Common causes of increased vascular permeability include infection, sepsis, aspiration, pulmonary contusions in trauma, blood transfusion (transfusion-related acute lung injury [TRALI]), and disseminated intravascular coagulation.6
PHYSIOLOGY OF PREGNANCY AND PULMONARY EDEMA
When compared with healthy nonpregnant adults, pregnant women exhibit many physiologic changes that favor the development of pulmonary edema. Normal pregnancy has been associated with increased intravascular volume, increased vascular permeability, a lower serum colloid osmotic pressure, and a low colloid osmotic pressure to left ventricular end-diastolic pressure gradient.5 Even in the absence of pathology, these physiologic changes can predispose parturients to develop pulmonary edema. When approaching term, pregnant women have also been reported to exhibit a mild decrease in systolic and diastolic functions.7 Fortunately, most parturients exhibit good underlying cardiac function and cardiovascular reserve which prevent the manifestations of pulmonary edema; however, one major or several minor insults or comorbidities can act to decrease that reserve resulting in decompensation and the development of pulmonary edema. Preeclampsia, chronic hypertension, obesity, tobacco use, and advanced maternal age are examples of many factors that can lead to the physiologic changes of pregnancy becoming pathologic.8
Pregnancy-Associated Cardiac Disease
One of the subgroups of obstetric patients at highest risk for developing pulmonary edema is the population with underlying structural or functional cardiac disorders. The cardiac dysfunction exhibited by these patients can be subdivided into three distinct groups: patients with (1) systolic dysfunction (impaired contractility, ejection fraction less than 45%); (2) diastolic dysfunction with resultant elevated filling pressures from abnormal ventricular myocardial relaxation; or (3) valvular disorders. It is not uncommon for some very high-risk patients to possess more than one of these subgroups. Common cardiac comorbidities identified in patients who develop pulmonary edema include those with valvular disease (aortic regurgitation and stenosis, mitral stenosis, and regurgitation) and cardiomyopathies (categorized as dilated, hypertrophic, ischemic, and peripartum).2 Interestingly, a significant percentage of women with underlying structural heart disease are diagnosed at the time of work-up for pulmonary edema.9 Pregnancy itself then may be viewed as a physiologic challenge test in which a subset of parturients with subclinical abnormalities fail. Thus, suspicion of an undiagnosed cardiac disorder should be raised in any pregnant patient in whom pulmonary edema develops. Similar thought also has been applied to hypertensive disorders of pregnancy where a higher lifelong risk of hypertension and other cardiovascular disorders have been identified following a diagnosis of preeclampsia.10,11
Preeclampsia
Preeclampsia affects approximately 5% to 6% of all pregnancies, and contributes significantly to maternal and fetal morbidity and mortality.12 It is reported in a small case series of preeclampsia that nearly 3% of women diagnosed with preeclampsia developed pulmonary edema (10 out of 345).13 This was primarily a postpartum phenomenon with 70% of cases occurring after delivery of the fetus. The pulmonary edema associated with preeclampsia has characteristics of both cardiogenic and noncardiogenic subtypes.4 Preeclampsia can lead to acute decompensated systolic or diastolic heart failure through elevations in afterload, cardiac ischemia, cardiac ventricular wall edema, and remodeling with fibrosis formation (Figure 8-1). Several recent studies using echocardiography and left ventricular tissue Doppler indices describe impaired myocardial contractility, especially in preterm preeclampsia. Likewise, studies on maternal diastolic dysfunction demonstrate mild to moderate range global diastolic impairment and segmental dysfunction of myocardial relaxation in both term and preterm preeclamptic patients.14 Preeclampsia can also increase vascular permeability leading to interstitial edema through diffuse endothelial injury.15 The mechanism by which preeclampsia leads to endothelial dysfunction and vascular capillary leakage of fluid is currently not well elucidated. Several hypotheses exist, including a role for cardiotonic bufodienolides such as marinobufagenin, other angiogenic factors, and proapoptotic mechanisms all acting to disrupt tight junction proteins leading to vascular leak. Pulmonary edema is one of the criteria sufficient to elevate a patient’s diagnosis to preeclampsia with severe clinical features even in the absence of blood pressure values in the severe range. Pulmonary edema is one of the major causes of maternal morbidity in the preeclamptic patient.8 Women at the highest risk of pulmonary edema in the setting of preeclampsia are those who have received a large amount of intravenous (IV) fluid (5000 mL or greater), those who develop HELLP syndrome (4.3%-15% of preeclamptics with pulmonary edema), or those who present with, or progress to, eclampsia (5%-33% of preeclamptics with pulmonary edema).16
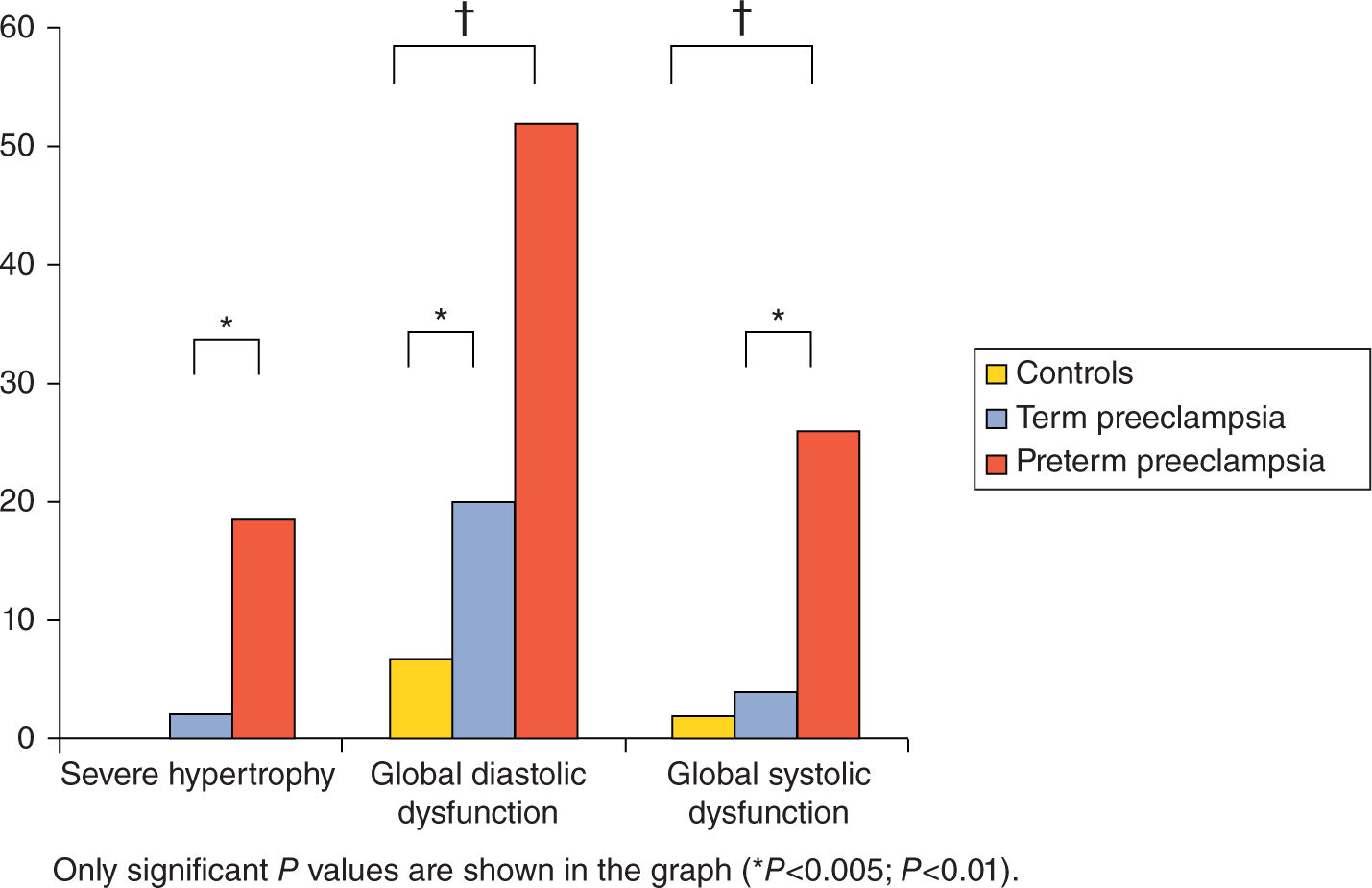
FIGURE 8-1. Prevalence of left ventricular severe hypertrophy, global diastolic dysfunction, and global systolic dysfunction in preterm and term preeclampsia cases and controls. Reproduced with permission from Melchiorre K, Thilaganathan B: Maternal cardiac function in preeclampsia, Curr Opin Obstet Gynecol. 2011 Dec;23(6):440-447.14
Tocolytic Therapy
The incidence of prematurity in the developed world is approximately 5% to 18% and continues to present a major public health problem. The vast majority of preterm births (70%) are because of spontaneous premature labor.17 Tocolytic therapy is commonly employed to delay delivery 24 to 48 hours to allow the administration of maternal corticosteroids in an effort to aid fetal lung development and promote maturity.18 The most common medications prescribed for tocolysis are β-2 agonists (eg, terbulaline), calcium channel blockers (eg, nifedipine and nicardipine), indomethacin, and magnesium sulfate, all of which have been implicated in the risk for developing pulmonary edema.
Beta-adrenergic receptor agonists such as terbutaline are routinely administered via IV and subcutaneous routes for tocolysis. Transient minor side effects including tachycardia, tremors, shortness of breath, and chest discomfort have been reported in 15.4% of 9359 parturients who received terbutaline in a large retrospective examination of an administrative database.19 Fortunately, more serious side effects presented in only 12 patients (0.12%). These included pulmonary edema in nine (<0.1%), peripartum cardiomyopathy in two, and ventricular arrhythmia in one. The majority of these nine women developed pulmonary edema in the setting of other comorbidities including preeclampsia or multiple gestations and/or concomitant administration of another class of tocolytic agent.
Calcium channel blocking agents, primarily used in the general medical population for control of blood pressure, also possess favorable tocolytic properties. Nifedipine is an orally administered medication, whereas nicardipine can be administered orally or parenterally, and both agents have been implicated in the development of pulmonary edema during pregnancy. Nicardipine administration specifically has been found to be independently associated with pulmonary edema. Serena et al. identified and described four cases of acute pulmonary edema in women without underlying cardiac disorders who were receiving IV nicardipine for tocolysis. In each case, intensive care unit admission was necessary because of the clinical severity of the patient’s symptoms.20 It is unclear whether nifedipine administration is an independent risk factor for the development of pulmonary edema. Xiao et al. report in a retrospective case-control study of 28 cases of pulmonary edema in the setting of preterm labor that nifedipine alone did not increase the odds of developing pulmonary unless it was concurrently administered with magnesium sulfate. However, several case reports and a larger case-control study by Ogunyemi concluded that nifedipine administered as a lone tocolytic agent is sufficient to increase risk of pulmonary edema.21,22 A likely mechanism for calcium channel blocker-related pulmonary edema is the combination of negative inotropy and impaired diastolic filling time from reflex tachycardia. Furthermore, calcium channel blockers have a greater vasodilatory effect on precapillary versus postcapillary blood vessels, which also increases interstitial fluid accumulation.22 Despite these findings, nifedipine and nicardipine may be safer with regard to pulmonary edema than other tocolytic agents and are often the agents of choice in woman at highest risk of pulmonary edema.
Magnesium sulfate is one of the most commonly administered medications in obstetrics for preterm labor tocolysis, eclamptic seizure prophylaxis, and recently to mitigate the impact of cerebral palsy on the preterm fetus. The mechanism of action of magnesium for any of these indications is largely unknown. Unfortunately, magnesium sulfate administration has been strongly associated with the development of pulmonary edema in preterm labor population. The incidence reported in retrospective analyses range from 6.7% to 8.5%, which represents a substantially higher incidence than in most other pregnant cohorts.23 Most often the benefits of magnesium administration to the parturient outweigh risks; however, health care providers who order or administer this medication should recognize the very real potential for adverse events, and be vigilant in monitoring for the signs and symptoms of serious cardiopulmonary complications.
Another patient subgroup routinely exposed to tocolytic therapy is those undergoing open fetal surgery.24 Tocolytic agents are administered prophylactically in an attempt to relax the uterus for optimal surgical conditions and to prevent the onset of preterm labor following these high-risk procedures. Specific tocolytic regimens vary, but they often entail the administration of multiple agents concurrently. During the procedure, IV nitroglycerin is commonly employed as a short acting agent if additional uterine relaxation is required. Patients undergoing open fetal surgery may be at a significantly higher risk of developing pulmonary edema than other populations. One study at a single tertiary care center reported an incidence of 23%, in contrast to the overall incidence of 0.08% in the obstetric population.24 Furthermore, it has been reported that patients exposed to IV nitroglycerin during open fetal surgery develop more severe disease and take significantly longer to recover. This specific high complication rate adds to the already challenging management of patients receiving fetal surgery.
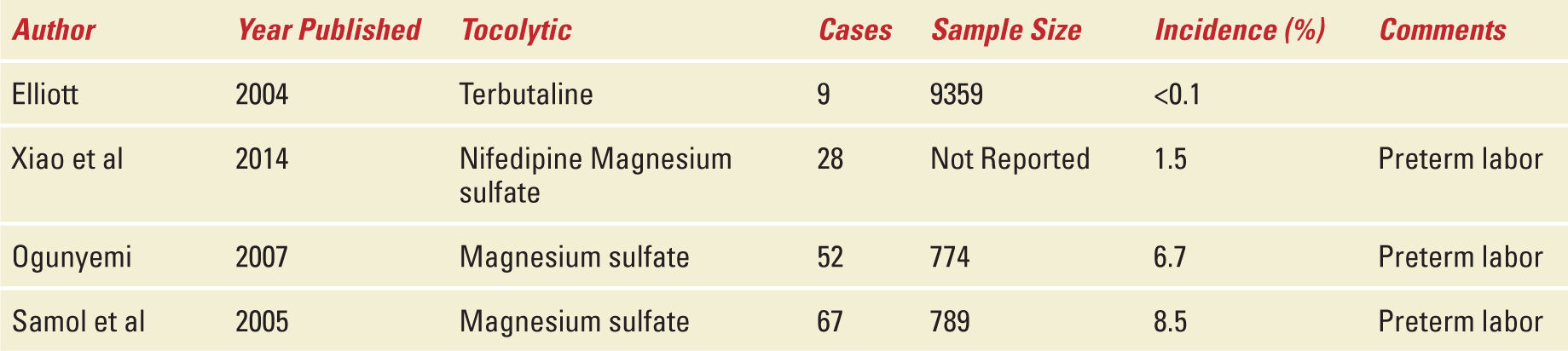
It is likely that the development of pulmonary edema related to the use of tocolytic agents is mainly driven by the underlying disease (eg, preterm labor with subclinical chorioamnionitis resulting in a leaky vasculature) and not by the tocolytic agent itself.Table 8-2 shows the incidence of pulmonary edema associated with the use of different tocolytic agents.
TABLE 8-2 | Incidence of Pulmonary Edema Associated with Tocolytic Therapy |
Volume Overload
Pregnant patients have the potential to develop hydrostatic pulmonary edema secondary to iatrogenic fluid overload, especially in the intrapartum and postpartum periods. Indeed, in women without other identifiable risk factors for pulmonary edema, iatrogenic fluid overload is the most common etiology.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


