Chapter 488 Principles of Treatment
The best chance for cure of cancer is during the initial course of treatment; the cure rates for patients with recurrent disease are much lower than those for patients with primary disease. All patients with cancer should be referred to an appropriate specialized center as soon as possible when the diagnosis of cancer is suspected. All such centers in North America are identified on the Children’s Oncology Group website (www.childrensoncologygroup.org) and on the National Cancer Institute cancer trials website (www.clinicaltrials.gov). The remarkable increases in cure rates for childhood malignancies since the 1980s would not have occurred without the collective participation of patients and their physicians in clinical research programs at these centers. In the USA, the National Cancer Institute’s Clinical Trials Cooperative Groups Program has been associated with a >80% reduction in the incidence of mortality due to cancer among children <15 yr of age despite an overall increase in cancer incidence during this interval (Fig. 488-1). This remarkable achievement represents the effects of an effective multimodal, multi-institutional, multidisciplinary collaboration.
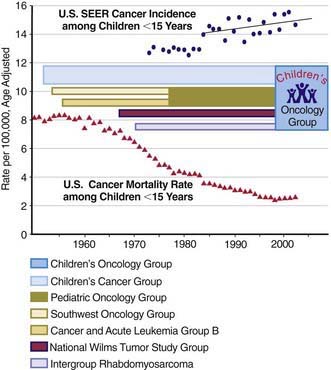
(Incidence and mortality rate data from Ries LAG, Eisner MP, Kosary CL, et al, editors: SEER Cancer Statistics Review, 1975-2002, Bethesda, MD, National Cancer Institute; http://seer.cancer.gov/csr/1975_2002/, based on November 2004 SEER (Surveillance Epidemiology and End Results) data submission, posted to the SEER website 2005. The mortality rate data are national rates, and the incidence data are derived from the SEER program, representing about 15% of the USA.)
The most current information on treatment of all types of childhood cancer is available in the PDQ (Physician Data Query) on the National Cancer Institute website (www.cancer.gov/cancertopics/pdq/pediatrictreatment).
A Multimodal, Multidisciplinary Approach
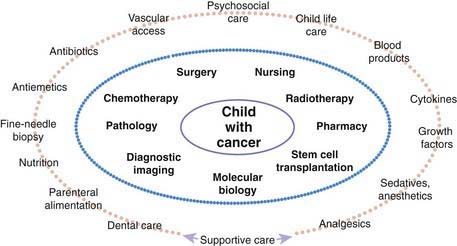
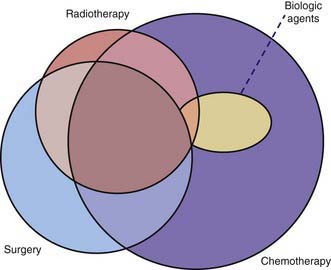
Many pediatric subspecialties are involved in the evaluation, treatment, and management of children with cancer, including provision of primary therapy and supportive care services (Fig. 488-2). More than 2 of the primary modalities are often used together, with chemotherapy being the most widely used, followed, in order of use, by surgery, radiation therapy, and biologic agent therapy (Fig. 488-3).
The leukemias that occur in childhood usually are managed with chemotherapy alone, with a small proportion of patients receiving cranial or craniospinal radiation therapy to prevent or treat overt central nervous system (CNS) leukemia. Children with non-Hodgkin lymphoma also are treated with chemotherapy alone, with the exception of radiation therapy for CNS involvement. Localized therapy with surgery or irradiation, or both, is an important component of treatment of most solid tumors, including Hodgkin lymphoma, but systemic multiagent chemotherapy usually is necessary because tumor dissemination generally is present even if it is undetectable. Chemotherapy alone usually is not adequate to eradicate gross residual tumors. Hence, it is not unusual for children with malignant tumors to require treatment with all three modalities (see Fig. 488-3). Unfortunately, most treatments that are effective in children with cancer have a narrow therapeutic index (a low ratio of efficacy to toxicity). The acute and chronic adverse effects of these treatments can be minimized but not entirely avoided.
Over the past 15 yr, biologic agent therapy has become an important modality in a few childhood cancers (see Fig. 488-3). This type of treatment generally refers to immunotherapy, biologic response modifiers, or endogenously occurring molecules that have therapeutic effects in supraphysiologic doses. Examples are retinoic acid therapy in acute promyelocytic leukemia, monoclonal antibody therapy for neuroblastoma and certain non-Hodgkin lymphomas, imatinib mesylate for chronic myelogenous and Philadelphia chromosome–positive leukemias, and radioactive metaiodobenzylguanidine therapy for neuroblastoma.
Development of selective, highly effective therapy for cancer in both children and adults had been hindered by a lack of understanding of the molecular mechanisms that underlie malignant transformation. De novo or acquired resistance to chemotherapy and radiation therapy remains an obstacle to cure. Ongoing discoveries of molecular and cellular mechanisms that explain the cancer process have led to increasingly specific antineoplastic therapies, generally referred to as molecularly targeted therapies. Their most prominent feature is a relative lack of normal tissue toxicity, such that the additional therapeutic benefit occurs with minimum additional toxicity. Many of the new biologic agent therapies, such as imatinib and rituximab, fall into this category (Table 488-1). Complementary and alternative remedies are increasingly being provided by parents to their children with cancer, with or without knowledge of the medical professionals entrusted with the child’s care (Chapter 59). Many of these have not been evaluated by rigorous testing and most are ineffective; some are toxic or interfere with the metabolism of other drugs. Although dramatic advances in the discipline have reduced the empiricism of therapy for cancer, much remains to be discovered.
Table 488-1 PROTEIN TYROSINE KINASE INHIBITORS AND MONOCLONAL ANTIBODIES
| AGENT | KINASE | MALIGNANCY |
|---|---|---|
| Imatinib | BCR-ABL | CML Philadelphia chromosome positive ALL |
| PDGFRα | Hypereosinophilic syndrome Systemic mastocytosis | |
| PDGFRβ | CMML | |
| cKIT | Systemic mastocytosis Gastrointestinal stromal tumor | |
| Dasatinib | BCR-ABL | CML Philadelphia chromosome-positive ALL |
| Nilotinib | BCR-ABL | CML Philadelphia chromosome-positive ALL |
| Gefitinib | EGFR | Non–small cell lung cancer |
| Erlotinib | EGFR | Non–small cell lung cancer |
| Trastuzumab | ERBB2/HER-2 | Breast cancer |
| Cetuximab | EGFR | Non–small cell lung cancer Squamous cell cancer of head/neck |
| Bevacizumab | VEGFR-1, -2 | Non–small cell lung cancer Breast cancer Renal cell carcinoma Colorectal cancer Glioblastoma |
ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CMML, chronic mono myelogenous leukemia.