Chapter 237 Principles of Antiviral Therapy
Antiviral chemotherapy typically involves a delicate interplay between host cellular functions and viral targets of action. Many antiviral agents exert significant host cellular toxicity, a limitation that has hindered antiviral drug development. In spite of this limitation, a number of agents are licensed for use against viruses, particularly herpesviruses, respiratory viruses, and hepatitis viruses. In addition to licensed antivirals and recommended regimens (![]() see Table 237-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), several studies are actively enrolling children for evaluation of novel antiviral therapeutic approaches. These studies are funded by the National Institutes of Health and administered through the Collaborative Antiviral Study Group (CASG), and up-to-date information is available about active clinical protocols at the CASG web page (http://medicine.uab.edu/Peds/CASG/).
see Table 237-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), several studies are actively enrolling children for evaluation of novel antiviral therapeutic approaches. These studies are funded by the National Institutes of Health and administered through the Collaborative Antiviral Study Group (CASG), and up-to-date information is available about active clinical protocols at the CASG web page (http://medicine.uab.edu/Peds/CASG/).
Table 237-1 CURRENTLY LICENSED ANTIVIRAL DRUGS*
| ANTIVIRAL | TRADE NAME | MECHANISM OF ACTION |
|---|---|---|
| Acyclovir | Zovirax | Inhibits viral DNA polymerase |
| Adefovir | Hepsera | Nucleotide reverse transcriptase inhibitor |
| Amantadine | Symmetrel | Blocks M2 protein ion channel |
| Cidofovir | Vistide | Inhibits viral DNA polymerase |
| Famciclovir | Famvir | Inhibits viral DNA polymerase |
| Fomivirsen | Vitravene | Phosphorothioate oligonucleotide inhibits viral replication via antisense mechanism |
| Foscarnet | Foscavir | Inhibits viral DNA polymerase and reverse transcriptase at pyrophosphate-binding site |
| Ganciclovir | Cytovene | Inhibits viral DNA polymerase |
| Idoxuridine | Herplex | Inhibits viral DNA polymerase |
| Interferon-α | Intro-A (interferon-α 2b) Roferon-A (interferon-α 2a) Infergen (interferon alfacon-1) | Produces multiple effector proteins that exert antiviral effects; also directly interacts with immune system components |
| Interferon-α 2b plus ribavirin | Rebetron | Not established |
| Lamivudine | Epivir | Inhibits viral DNA polymerase and reverse transcriptase |
| Oseltamivir | Tamiflu | Neuraminidase inhibitor; interference with de-aggregation and release of viral progeny |
| Pegylated interferon | PEG-Intron (α 2b), Pegasys (α 2a) | Same as interferon |
| Penciclovir | Denavir | Inhibits viral DNA polymerase |
| Ribavirin | Virazole, Rebetol, Copegus | Interference with viral messenger RNA |
| Rimantadine | Flumadine | Blocks M2 protein ion channel |
| Trifluridine | Viroptic | Inhibits viral DNA polymerase |
| Valacyclovir | Valtrex | Same as acyclovir |
| Valganciclovir | Valcyte | Same as ganciclovir |
| Vidarabine | Ara-A | Inhibits viral DNA polymerase (and to lesser extent, cellular DNA polymerase) |
| Zanamivir | Relenza | Neuraminidase inhibitor; interference with de-aggregation and release of viral progeny |
| FDA-APPROVED COMBINATION THERAPIES | ||
| Interferon-α 2b + ribavirin | Rebetron (Intron-A plus Rebetol) | |
| Interferon-α 2a + ribavirin | Roferon-A + ribavirin | |
| Pegylated interferon-α 2b + ribavirin | PEG-Intron + Rebetol | |
| Pegylated interferon-α 2a + ribavirin | Pegasys + Copegus | |
* See Chapter 268 for antiretroviral drugs.
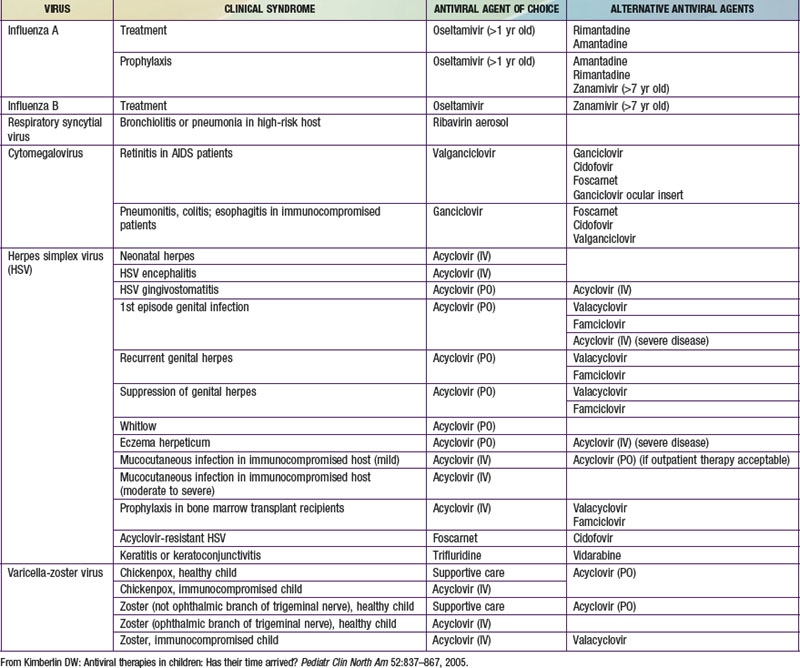
In making the decision to commence antiviral drugs, it is important for the clinician to obtain appropriate diagnostic specimens, which can help clarify the antiviral of choice. The choice of a specific antiviral is based on the recommended agent of choice for a particular clinical condition, pharmacokinetics, toxicities, cost, and the potential for development of resistance (Table 237-2). Intercurrent conditions in the patient, such as renal insufficiency, should also be considered. Clinicians must monitor antiviral therapy closely for adverse events or toxicities, both anticipated and unanticipated.