Chapter 225 Principles of Antifungal Therapy
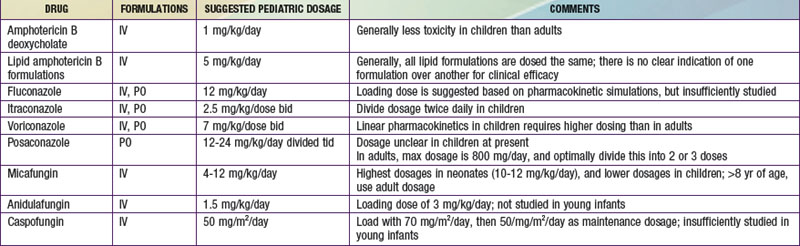
Due to advances in aggressive antineoplastic agents and organ transplantation, invasive fungal infections are a major cause of morbidity and mortality in children. Fortunately, the therapeutic armamentarium for invasive fungal infections has markedly increased since the turn of the century (![]() See Table 225-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
See Table 225-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).