Primary Care and Adolescent Medicine
Michele S. Duke
Anna Wheeler Rosenquist
Bradley Monash
Paritosh Prasad
Shannon E. Scott-Vernaglia
Breast-feeding
Breast-feeding Rates United States, 2001
(Pediatrics 2005;115:496)
70% initiate, 33% at 6 mo, 18% at 1 yr of age
Goal for Healthy People 2010: 75% initiate, 50% at 6 mo, 25% at 1 yr of age
Infant Benefits
↑ immunity: ↓ meningitis, bacteremia, UTI, AOM, diarrheal illness, URI, NEC
↓ postneonatal infant mortality; decreased SIDS, incidence of Type I IDDM, lymphoma, leukemia, Hodgkin disease, obesity, hyperlipidemia, asthma
Enhanced performance on tests of cognitive development
Maternal Benefits
Decreases postpartum bleeding, risk of breast CA, and ovarian CA
May decrease incidence of osteoporosis and hip fractures after menopause
Earlier return to prepregnancy weight
Contraindications
Mother on contraindicated medications; tables available (Pediatrics 2001;108:776)
Infant with classic galactosemia (galactose 1-phosphate uridyltransferase deficiency)
Mother with HTLV
Mother receiving radioactive isotopes until cleared, antimetabolites or chemotherapeutic agents, or those using drugs of abuse
Herpes simplex virus lesions of the breast (may use other breast if unaffected)
HIV infection (must do risk-benefit analysis in developing countries because of lack of clean water supply, availability of formula, and frequency of dehydrating illnesses)
NOT Contraindicated
Maternal Hep B surface antigen positive, Hep C infection (Ab or RNA positive)
Maternal carriage of CMV (not recent converters), isolated maternal fever
Selected AAP Breast-feeding Recommendations
Vitamin D supplementation
Begin w/i 2 mo; 200 IU daily
D/c when daily consumption of Vitamin D fortified formula or milk >500 mL
Frequency of feeding
8–12 times daily during initiation; 6–8 times daily when well established
Complementary foods and liquids
No water or juice needed under 6 mo; no cow’s milk until age 1 yr
Introduce complementary iron rich foods at 4–8 mo
Follow up visits
Check weight and breast-feeding at 3–5 d and 10–14 d
Special considerations for low-birth-weight, prematurity, or if risk for hemolysis
Breast-feeding Support
Lactation consultant www.ilca.org, La Leche League www.llli.org
Cow’s Milk Protein Allergy
See Allergy section
Reflux
See GI section
Sudden Infant Death Syndrome (SIDS)
Definition
(Pediatr Rev 2007;28:209)
Sudden unexplained death of an infant younger than 1 yr of age.
Usually previously healthy infant; cause of death unexplained despite investigation
Epidemiology
2500 infants yearly in the U.S.; Male to female ratio 3:2
Rate ↓ from 1.2 to 0.57 deaths per 1000 live births from 1994 to 2002
Third leading cause of death in infancy, top cause of death in 1–12 mo olds
Risk Factors
Prone and side sleeping positions, soft bedding, overheating
Maternal smoking during pregnancy and environmental tobacco smoke
Inadequate prenatal care, young maternal age, prematurity or low-birth-weight
African American or Native American heritage (2–3× general population risk)
Family with one SIDS death has 2%–6% risk of a second SIDS death (see below)
Pathophysiology: Proposed Mechanisms
Rebreathing theory: prone infants trap exhaled CO2 around face, ↓ arousal: Some SIDS infants w/brainstem w/5HT-R abn at ventral medulla; ↓ arousal resp to hypercarbia and hypoxia
Some SIDS infants w/polymorphisms in 5HT transporter gene w/ ↓[5HT] at synapse
Other genes related to QT prolongation and autonomic nervous system development
Differential Diagnosis
Sepsis, PNA, cardiomyopathy, congenital heart dz, arrhythmia, prolonged QT, accidental or nonaccidental trauma, suffocation, and inherited metabolic disorders
Risk Reduction
Supine sleep position at all times (remind 2° caregivers). Side unacceptable.
Firm crib mattress covered w/single sheet, blanket tucked in on 3 sides, below waist
Decrease tobacco exposure
Pacifier use confers protection (90% ↓ risk). Begin after breastfeeding established.
Avoid bed sharing
Skull Deformities
(Clin Pediatr (Phila) 2007;46:292; Pediatrics 2003;112:199)
Deformational Plagiocephaly
(J Craniofac Surg 2007;18:85)
Definition: Plagiocephaly: Greek. “Oblique head.”
Deformational (positional) plagiocephaly: benign positional molding, flattening of occiput 2/2 gravitational forces on nml malleable skull
Epidemiology: 1 in 68 have cranial asymmetry 2/2 deformational plagiocephaly (DP)
DP has ↑’ed 6-fold since institution of AAP’s “Back to Sleep” campaign
Etiology assoc w/supine pos, ↓ time on abd, ↑ use car seats/carriers, unvaried feed position
Can also result from or be exacerbated by congenital torticollis or visual deficits
Clinical presentation: Hx of symmetric head at birth
Flattening of occiput, anter displacement of ipsilateral ear, forehead, and cheek
Head takes on a parallelogram shape
Differential: Lambdoid craniosynostosis presents similarly; Rare: incidence 1 in 300,000
In contrast to DP, ear posteriorly displaced; head takes on a trapezoidal shape
Palpable bony ridge at lambdoid suture, between occipital and parietal bones
Diagnosis, Prevention, and Treatment
Imaging not needed; must rule out visual deficits, congen torticollis as cause
Positional: ↑ “Tummy time,” Δ sleep position (promote alt side of head against bed; pt will fall asleep facing area of activity), alter feeding position
Physical therapy: neck stretching and strengthening exercises
Helmet therapy: if no improvement after 2–3 mo of above interventions
Formal criteria for helmet involve transcranial diagonal diameter
Decision to refer: Assessment tool available at www.cranialtech.com
Craniosynostosis
Premature closure of sutures causing skull deformity
Epidemiology: Craniosynostosis is uncommon, seen in 1 in 2000
Etiology: Poorly described genetic and environmental factors
Clinical presentation: Frequently has history of abnormal head shape since birth
Head shape depends upon which sutures fuse prematurely
Head growth proceeds in direction perpendicular to prematurely fused sutures
Resulting head shape can lead to ↑ ICP, cognitive and neurologic deficits
Can be syndromic: Alagille, Apert, Cornelia de Lange, Crouzon, Treacher-Collins
Diagnosis and treatment
Immediate referral to neurosurgeon before imaging is appropriate if suspected
Treatment is with helmet and/or surgery
Neonatal Hyperbilirubinemia
Introduction
(Pediatr Rev 2006;27:443; Pediatrics 2004;114:297)
Jaundice = yellowing of skin, conjunctiva, and mucous membranes 2/2 deposition of bilirubin, which is produced from the breakdown of hemoglobin (Hgb)
Occurs in 60% of healthy FT infants; 10% develop severe jaundice (>17 mg/dL)
Appears w/cephalocaudal progression (face to trunk to palms and soles): Face = 5 mg/dL, chest = 10 mg/dL, abdomen = 12 mg/dL, and palms/soles >15 mg/dL. Not visible if <4 mg/dL. If above nipple line, level likely <12 mg/dL.
Need confirmation via transcutaneous or serum bilirubin measurement
Acute bilirubin encephalopathy (ABE) is the acute manifestation of bilirubin toxicity
Early ABE: Lethargy, poor feeding, high-pitched cry, hypotonia
Late ABE: Hypertonia; backward arching of neck (retrocollis) and trunk (opisthotonos); seizures, apnea, fever
Kernicterus: permanent sequelae of bili deposition in basal ganglia & brainstem.
MR, athetoid CP, upward gaze paralysis, hi-freq hearing loss/deafness, enamel dysp
Bilirubin Metabolism
(Pediatr Rev 2006;27:443)
Plasma: Hgb degraded by heme oxygenase and biliverdin reductase to unconj bilirubin, bound to albumin. Unconj bili fat soluble; able to cross the blood–brain barrier
Liver: unconj bili converted via glucuronosyltransferase (UGT-1) to conj bilirubin, excreted into bile via MRP2 transporter. Conj bili water soluble, can be excreted in urine, cannot cross blood–brain barrier to produce neurotoxicity.
Intestine: conj bili degraded by bacteria to urobilinogen and excreted in feces. Newborn gut sterile precludes breakdown; conj bili hydrolyzed back to unconj form and absorbed into enterohepatic circulation (EH circulation).
Pathophysiology
(Pediatr Rev 2006;27:443)
Hyperbilirubinemia 2/2 ↑ production, ↓ conjugation, or impaired excretion bilirubin.
↑ Bili production (unconjugated)
Hemolytic (>6% reticulocyte count, hemoglobin <13, hepatosplenomegaly)
Coombs (+): ABO, Rh, and minor antigen incompatibility
Coombs (-): RBC memb defects (spherocytosis), enzyme def (pyruvate kinase, G6PD), Hgb defects (SCD, thal), drugs (streptomycin, Vit K)
Nonhemolytic (normal reticulocyte count and hemoglobin)
Extravascular blood: Cephalohematoma, bruising, CNS bleed
Intravascular blood = Polycythemia (High Hb/HCT): 2/2 delayed cord clamping, fetal-maternal xfusion, twin-twin xfusion, maternal DM or smoking, high altitude
Intestinal = ↑ EH circ; ↓ stooling, CF, Hirschsprung, pyloric stenosis, obstruct
↓ Bili conj (unconj): Breast milk Jaundice, Hypothyroid, Gilbert and Crigler-Najjar
Impaired bilirubin excretion (conjugated)
Biliary obstruction: Biliary atresia, choledochal cyst, 1° sclerosing cholangitis, gallstones, Dubin-Johnson, and Rotor syndromes
Metabolic Disease: α-1 antitrypsin def, CF, galactosemia, glycogen storage dz, Gaucher, Wilson, Niemann-Pick, Genetic dz, Trisomy 21 and 18, Turner
Infection: UTI, sepsis, idiopathic neonatal hepatitis, Hep B, TORCH
↓ Albumin binding (unconj): low albumin, meds (CTX, sulfa, steroids), acidosis
Physiologic Jaundice
(Pediatr Rev 2006;27:443)
Includes (1) Breast-feeding jaundice and (2) breast milk jaundice. 2/2 multi factors:
Newborn relatively polycythemic, which is resolved by hemolysis
Neonatal erythrocytes have shorter lifespan (80 vs 120 d) w/inc turnover
Immature liver: (1) ↓ glucuronyl transferase and (2) ↓ uptake of unconj bilirubin
↑ EH circ: Sterile neonatal gut doesn’t degrade conj bili, reverts and is reabsorbed
Colostrum: Small vol leads to weight loss and slow passage of bili-rich meconium
Breast-feeding jaundice
Early onset; peaks DOL 3–4: 2/2 relative caloric depriv, mild dehyd, and delayed passage of mec; Rx: inc freq feeds (10×/d w/formula suppl as needed).
Breast milk jaundice
Late onset; peaks DOL 6–14; jaundice begin ↓ at 2 wk, can be ↑ up to 3 mo
2/2 breast milk substances (B-glucuronidases and nonesterified fatty acids), which inhibit normal bilirubin metabolism; actual causal substance unknown
Rx: interrupt breast-feeding ∼2 d (to ↓ bili level, pump in btw), then resume.
Pathologic Jaundice
(Am Fam Physician 2002;65:599)
Any jaundice w/i 1st 24 hr of life or after 14 d, any rapid rise Tbili (>5mg/dL/d), any Tbili >17 mg/dL in FT newborns, or any evidence of underlying illness.
Risk Factors for Development of Severe Hyperbilirubinemia
(Pediatrics 2004;114:297)
Predischarge bili level in the high-risk zone, jaundice in 1st 24 hr, ABO incomp or known hemolytic dz, GA <37 wk, prev sibling Rx’d w/phototherapy, cephalohematoma/bruising, exclusive breast-feeding, East Asian race. Minor factors: macrosomic infant of diabetic mother, maternal age >25 yr, males, albumin <3.0
Normogram for designation of risk (Pediatrics 1999;103:6)
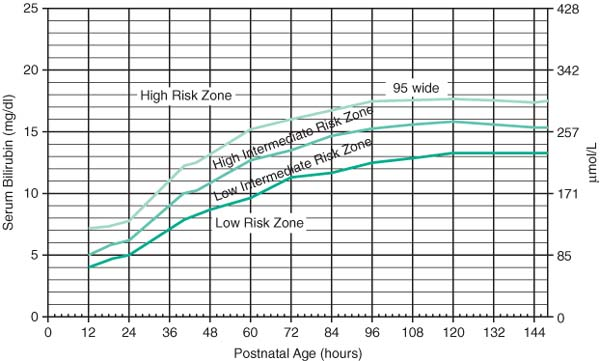 |
Evaluation
(Am Fam Physician 2002;65:599)
Physical Exam
Cephalocaudal progression (face = 5, chest = 10, abd = 12, palms & soles >15)
Bruises, pallor, petechiae, hepatosplenomegaly, weight loss, and dehydration
Labs: Tbili/Dbili, blood type, direct Ab test (Coomb), CBC/diff/Retic/smear
Consider G6PD, thyroid fxn tests, galactosemia screen, CF screen, albumin, U/A and cx, and or full sepsis evaluation (blood cx and LP as well)
Can use online calculators using Bhutani nomogram; www.bilitool.org
Rx of Unconjugated Hyperbili
(Pediatrics 1999;103:6; Pediatrics 2004;114:297; J Peds 1998;133:705)
Phototherapy (PTX): light in blue–green spectrum (wavelength 425–490 nm) convert unconj bili to water-soluble form excreted in bile and urine w/o conjugation
Family history of porphyria is an absolute contraindication to phototherapy
If bili does not fall or continues to rise despite phototherapy, hemolysis is likely
Serum albumin <3.0 lowers threshold to start phototherapy
Stop phototherapy when bilirubin is <13–14 mg/dL
No need to delay discharge to check rebound bilirubin level. If hemolytic dz, or if PTX is d/c’d before infant is 4 d old, check rebound bili level 24 hr after d/c.
Guidelines for Phototherapy in Hospitalized Infants ≥35 Weeks (AAP Guidelines 2004)
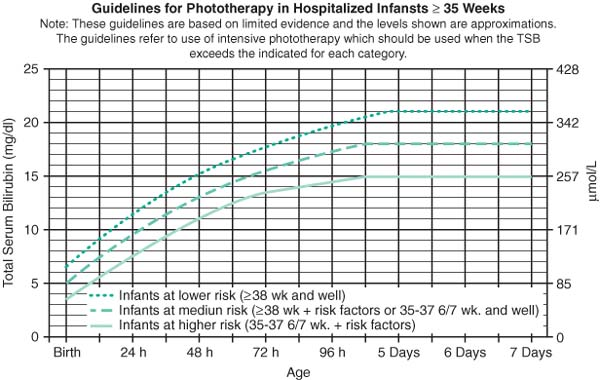 |
Exchange transfusion: removes bilirubin and damaged erythrocytes from circulation
Reaching exchange transfusion levels (bili ≥25 mg/dL) is a medical emergency!
In isoimmune hemolytic dz, Rx w/IVIG (0.5–1g/kg over 2 hr) if bili is rising despite intensive PTX or if bili level is w/i 2 mg/dL of the exchange level.
Calculate “bilirubin/albumin” ratio to determine need for exchange transfusion:
Infants ≥ 38 weeks: 8.0; if higher then concerning
Infants 35–37.9 weeks and well, or if hemolytic disease: 7.2
Infants 35–37.9 weeks and high risk, or if hemolytic disease: 6.8
Guidelines for Exchange Transfusion in Infants ≥35 Weeks (AAP Guidelines 2004)
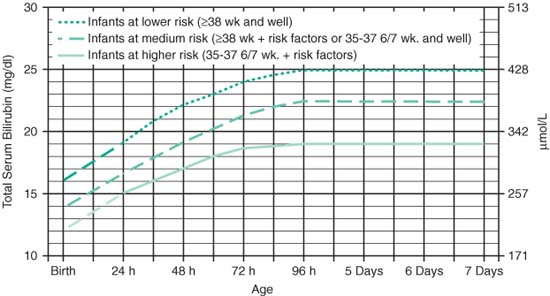 |
Pharmacology
Phenobarbital and ursodeoxycholic acid lower bili levels by facilitating bile flow
Tin-mesoporphyrin—inhibits production of heme oxygenase (not FDA approved)
Colic
See GI section
Normal Growth
(Nelson Textbook of Peds, 18th Ed. Saunders 2007. 70–74, 677, 2434)
(Pediatr Rev 2006 Jan;27(1):e1)
Term infants lose up to 10% of BW, then regain BW by 2 weeks.
BW doubles by 4–5 mo, and triples by 1 yr; height doubles by age 3–4.
Exclusively breast-fed infants smaller than formula-fed from 6–12 mo; resolves by 1 yr
From 3–10 yrs old, children grow ∼2.5 inches/yr.
Anterior fontanelle: normal size 20 ± 10 mm; closes at 9–18 mo
Posterior fontanelle: closes by 2 mo
Excessively large fontanelle: IUGR, hypothyroid, prematurity, Trisomy 13/18/21, hydrocephalus, achondroplasia, Apert syndrome, Cleidocranial dysostosis, cong. rubella, Hallermann-Streiff syndrome, hypophos, Kenny syndrome, osteogenesis imperfecta, pyknodysostosis, Russell-Silver syndrome, Vit D def rickets
Excessively small fontanelles: microcephaly, craniosynostosis, hyperthyroidism
Average Growth and Caloric Requirements
| Age | Average Weight Gain (g/d) | Length (cm/mo) | Head Circumference (cm/mo) | Daily Caloric Allowance (Kcal/kg/d) |
|---|---|---|---|---|
| Birth – 3 mo | 25 – 30 | 3.5 | 2.0 | 115 |
| 3 mo – 6 mo | 20 | 2.0 | 1.0 | 110 |
| 6 mo – 9 mo | 15 | 1.5 | 0.5 | 100 |
| 9 mo –12 mo | 12 | 1.2 | 0.5 | 100 |
| 1 yr – 3 yr | 8 | 1.0 | 0.25 | 100 |
| 4 yr – 6 yr | 6 | 3 cm/yr | 1 cm/yr | 90–100 |
Midparental Height 1
3 cm (instead of ± 5 inches) if using metric units
Boys: (paternal height in inches + maternal height in inches + 5)/2
Girls: (parental height in inches + maternal height in inches – 5)/2
Growth Charts
(Am Fam Physician 2003;68:879) (growth charts at www.cdc.gov/growthcharts)
Length should be measured via length-board; height measured via stadiometer
Head circ measured just above eyebrow and ears, across most prominent part occiput
Special growth curves available for: Trisomy 21, Prader-Willi, Williams syndrome, Cornelia de Lange syndrome, Turner syndrome, Rubinstein-Taybi syndrome, Marfan syndrome, achondroplasia, and very low BW infants <1500 g (use Infant Health and Developmental Program (IHDP) growth curves)
Failure to Thrive
(Pediatr Rev 2006 Jan;27(1):e1; Clin Fam Pr 2003;5:293; Pediatr Rev 2000;21:257)
Introduction
(Am F Phys 2003;68:879)
No standard dx criteria exist; Failure to thrive (FTT) most commonly defined as decel growth across 2 major percentile lines, or weight for age less than fifth percentile
1° etiology is malnut, 2/2 multi medical, behavioral, psychosocial, and environ causes
Malnutrition 1st decreases weight, then height then head circumference
Definition
(Am Fam Physician 2003;68:879)
Organic FTT: poor growth because of a known medical disorder
Nonorganic FTT: poor growth because of psychosocial factors, diagnosed by exclusion
Multifactorial FTT: poor growth 2/2 of both med & nonmed factors, most common
Gomez criteria for FTT severity: Compare current weight-for-age against expected weight for age (50th percentile)
<60% expected = severe FTT; 61%–75% expected = mod; 76%–90% = mild
Etiology
(Am Fam Physician 2003;68:879; Clin Fam Pr 2003;5:293; Pediatr Rev 2000;21:257)
Inadequate caloric intake
Incorrect formula prep or lactation inability, maladaptive feeding habits
Mech. feeding diff (anatomic, oral lesions), motor diff (oromotor dysfxn, CNS dz)
Familial dysfxn, disturbed parent–child relationship (neglect or hypervigilance)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


