Preinvasive Disease of the Lower Genital Tract
BRITT K. ERICKSON  KENNETH H. KIM
KENNETH H. KIM  MARK H. EINSTEIN
MARK H. EINSTEIN  WARNER K. HUH
WARNER K. HUH
INTRODUCTION
At the forefront of any discussion on preinvasive and invasive lesions of the lower genital tract is the Human Papillomavirus (HPV). Infection with HPV is necessary for the development of almost all preinvasive cervical and vaginal lesions, and it is present in roughly half of preinvasive vulvar disease. Moreover, HPV is the most commonly diagnosed sexually transmitted infection in the United States with an overall lifetime prevalence of nearly 80% and a point prevalence of over 40% (1, 2). Our knowledge surrounding HPV biology and epidemiology has increased exponentially in the past three decades, leading to improved screening modalities and recommendations as well as development of prophylactic vaccinations.
In this chapter, we will discuss the biology and epidemiology of HPV infections as they relate to cervical, vaginal, and vulvar carcinogenesis. Additionally, we will review clinical and HPV-associated risk factors for development of disease, the pathology of preinvasive lesions including cytology and histology, and discuss efficacy and impact of prophylactic HPV vaccination.
SECTION 1: HPV
Evidence for Causal Relationship
HPV was first proposed as a causative agent in the development of cervical cancer in the 1970s when Dr. Harald zur Hausen, a German physician and virologist, suggested that the same viral particles noted in genital warts may also be responsible for genital tract malignancies (3). This served to contrast the prevailing medical idea at that time that it was likely a herpes simplex virus that might have been the causative agent in cervical neoplasia. His work evolved and eventually in 1983, he isolated HPV type 16 and implicated its role in the development of cervical cancer (4). One year later, he had isolated HPV 18, thus discovering the two HPV types that today are associated with approximately 70% of all cervical cancers worldwide (5). For this body of important work, zur Hausen was awarded the 2008 Nobel Prize in Medicine.
In 1991, the International Agency for Research on Cancer (IARC) and the World Health Organization (WHO) concluded that, beyond a reasonable doubt, there is an association between HPV and cervical cancer (6). Though factors such as tobacco use, parity, contraceptive use, and sexual history may increase one’s risk for development of the disease, persistent high-risk HPV infection is indisputably the most important causative factor (7). The association between HPV and cervical cancer is far higher than the association between smoking and lung cancer (8). In fact, all steps, from HPV infection to cervical precancerous lesions, and from cervical carcinoma in situ to cancer, have been demonstrated in prospectively followed cohorts (9 – 11).
Classification of HPV
Papillomaviruses are double stranded DNA viruses that are members of the Alpha genus of the family Papovaviridae. Papillomaviruses are highly species specific and infect a wide range of vertebrate hosts. All papillomaviruses have regulatory, early (E) and late (L) genomic regions. Within a given host species, many types of papillomaviruses exist and this phylogenetic subdivision is determined by the extent of DNA relatedness. Specifically, the E6, E7, and L1 gene sequences must differ from one another by more than 10% to be classified as a distinct type, 2% to 10% to be a subtype, and 2% to be a variant of a subtype.
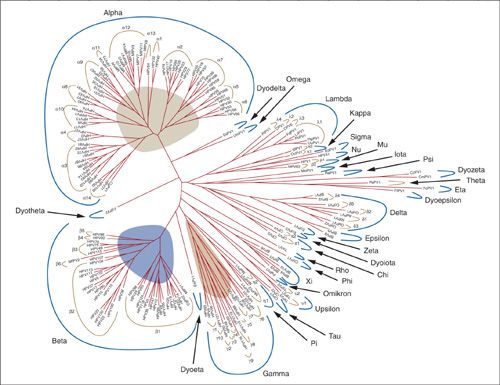
HPVs are epitheliotropic- infecting epithelial cells of the skin and mucous membranes and causing epithelial proliferation at the site of infection. HPV is currently divided into 120 distinct genotypes, and this list continues to expand (Fig. 7.1) (12). Over 40 types of HPV infect the anogenital tract (13). Traditionally, specific HPV types have been classified as high-risk types based on their potential to cause preinvasive and invasive disease. The most recent meeting of the IARC described 12 α-1 HPV types as high risk. These include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. Additionally, HPV 68, in the group α-2A, is categorized as probably carcinogenic (14).
Biology of HPV
HPV is a nonenveloped virus with a proteinaceous coat, which encases and protects the viral DNA. More specifically, the particle is composed of 72 capsomeres made of the viral proteins L1 and L2, also known as the major and minor capsid proteins, respectively. In addition to providing protection for the viral nucleic acid, the capsomeres also serve as the initial interaction site of the viral particle with the host cell.
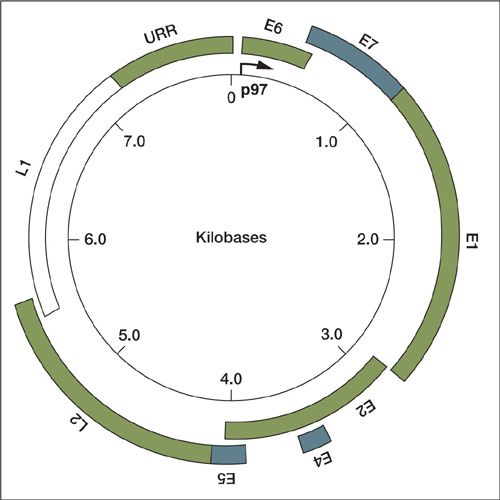
The HPV genome is circular, double stranded, and contains nearly 8,000 base pairs (Fig. 7.2). The overall organization of various HPV types is similar. The genome contains eight open reading frames, which are transcribed as a single polycistronic mRNA and through alternative splicing mechanisms and ribosomal scanning, this mRNA is translated into the eight proteins E1, E2, E4, E5, E6, E7, L1, and L2.
The HPV genome can be divided into three regions. The first region is the upstream regulatory region (URR), composed of nearly 1,000 base pairs. The URR does not code for proteins but contains binding sites for different cellular transcriptional activators and repressors, which then regulate the expression of the early viral genes (15). This region also contains binding sites for the viral proteins E1 and E2, which initiate viral replication and transcription (16). The URR is necessary for the regulation of gene expression, replication of the genome and packaging of virus particles.
FIGURE 7.1. Papillomavirus phylogenic tree.
Source: Reprinted with permission from Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010;401:70–79.
The URR primarily regulates the transcription of proteins in early infection including proteins E6, E7, E1, and E2. The late promoter is activated during the productive phase of the viral life cycle and results in transcription of the capsid proteins (L1 and L2) as well as E1, E2, E4, and E5.
HPV Lifecycle
Like other viruses, HPV must deliver its genome to the host cell and subsequently exploit the cellular machinery for its own purposes. HPV infects the host at sites of epithelial microtrauma, where the HPV particle can gain access to the actively proliferating basal cells of the epithelium (Fig. 7.3). The mechanisms of cell entry are complex and continue to be better elucidated (17). The HPV particle interacts with the cell surface via its major and minor capsid proteins (L1 and L2). α-6-Integrin had initially been suggested as a receptor; however, controversies exist regarding its specific involvement with HPV (18,19). Attachment receptors for HPV particles are likely heparan sulfate proteoglycans (HSPG), specifically syndecan-1, which is found in the extracellular matrix of epithelial cells (19). Laminim-5 may be another specific extra-cellular matrix receptor involved in binding and cell entry (20,21). Though the main target of HPV are the keratinocytes of the epithelium, HPV virus-like particles have also been shown to attach to cells important in immune function such as dendritic cells and Langerhans cells (22).
The binding of HPV to cell surface receptors in the basal cells over the basement membrane then initiates conformational changes in L2, which exposes additional binding sites (23,24). HPV then enters the cells via endocytosis. Most studies suggest clathrin or caveolin-dependent endocytosis (25), although internalization independent of these proteins has also been described (26).
Once inside the basal epithelial cells, the viral genome begins to replicate and maintains about 50 to 100 copies per cell. The life cycle of HPV is closely linked to the state of differentiation of the squamous epithelium of the natural host tissue. In normal squamous human epithelium, the basal layers (stratum basale) are the areas of active cell division. After division, the daughter cells migrate away from the basal cells (stratus spinosum) and no longer progress through the cell cycle. Instead, these terminally differentiated cells produce high–molecular-weight keratins until eventually the nuclear envelope breaks down and the cells become empty keratin-filled sacs (stratum corneum).
FIGURE 7.2. Schematic of genomic organization of HPV.
Source: Reprinted with permission from Wright TC, Ferenzy AF, Kurman RJ. Precancerous lesions of the cervix. In: Kurman RJ, ed. Blaustein’s Pathology of the Female Genital Tract. 4th ed. New York, NY: Springer-Verlag; 1994:229–241.
When the HPV-infected basal cell divides, the viral DNA segregates with the 2 daughter cells, 1 cell remains as part of the basal epithelium while the other migrates to the next layer. Because terminally differentiated cells of the upper epithelial layer contain little or no enzymes, in order to replicate, the virus causes the cell to reenter S phase, causing only partial instead of terminal differentiation. Thus the viral genome replication is synchronous with the cellular DNA replication. HPV infected cells migrate away from the basal layer and as the cells reach higher epithelial layers the late promoter is activated and late gene transcription and translation occur. There is high-level amplification of the viral genome in these layers and in the uppermost layer, DNA is packaged into capsids and the infectious virions are assembled. Typical HPV-associated cytopathic changes such as koilocytosis, multinucleation, and nuclear enlargement are due to the assembly of the viral particles in the upper epithelial layers. The epithelium is then shed and HPV particles are released, which can then infect a new host.
Oncogenic Proteins
The oncogenic potential of high-risk HPV is primarily attributed to the E6 and E7 proteins. E6 and E7 proteins differ in high-risk and low-risk types. These proteins do not have intrinsic enzymatic activities, but instead interact directly and indirectly with other cellular proteins affecting cell cycle regulation. In addition to their individual effects, their combined effects are likely synergistic. In transgenic mouse models, the combinations of E6 and E7 have resulted in particularly aggressive invasive cancers (27).
HPV E7
The E7 protein is responsible for immortalizing cells infected with HPV, primarily by affecting the cell’s transition from G1 into S-phase. E7 has a variety of targets including the retinoblastoma (Rb) protein family, histone deacetylases, cyclins, cyclin-dependent kinases (cdks) and cdk inhibitors (28). E7 is composed of 98 amino acids and is divided into 3 domains. CR1 is the amino terminus consisting of amino acids 1 to 20. The CR2 domain (amino acids 21 to 39) contains a sequence that binds E7 to retinoblastoma tumor-suppressor proteins. The CR3 domain (amino acids 40 to 98) contains zinc finger motifs that are essential for protein folding (29).
Rb proteins regulate the cell cycle by controlling the transition at the G1/S phase. Rb is hypophosphorylated and bound to E2F, a cellular transcription factor. In its bound state, Rb and E2F inhibit cellular proliferation. In normal cells, when cyclin-kinase complexes phosphorylate Rb, E2F is released and transcription of S phase genes occurs. E7 disrupts this process. The CR1 and CR2 domains of E7 bind to and degrade hypophos-phorylated Rb, disrupting the Rb-E2F complex (30,31). E2F is released, which results in the expression of S-phase genes.
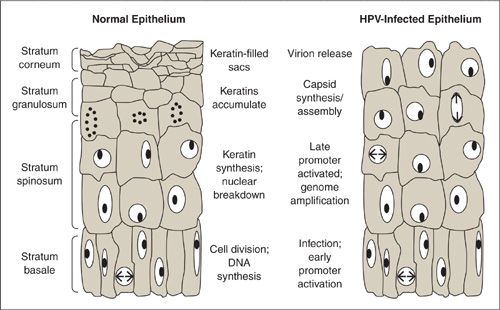
FIGURE 7.3. Diagram of normal epithelium and HPV-infected epithelium.
Source: Reprinted with permission from Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol 2006; 16(2):83–97.
The zinc finger region of E7 can interact with the class I histone deacetylases (HDACs). Through deacetylation of histones, HDACs induce chromatin remodeling (32). E7 binding to HDACs causes progression into S-phase thereby lengthening the life of the cell.
Independent of these effects, continuous activity of the E7 protein leads to increasing genomic instability, which leads to more dysregulated cell growth and eventually cancer. It has been observed that cells expressing E7 show irregularities in numerous centrosomes leading to aberrant mitotic spindle poles, thereby affecting chromosome number during replication (33). E7 expression is a late event in malignant transformation.
HPV E6
Like E7, E6 is a small 151 amino acid protein that induces important changes in the host cell. It also lacks endogenous enzymatic activity and binds to cell cycle-regulatory proteins, affecting life cycle and immortalization.
E6’s most notable effect is its ability to bind to p53. p53 is a transcriptional activator and is an important tumor-suppressor gene. p53 induces expression of genes involved in apoptosis and cell cycle arrest. p53 levels typically increase in response to stress, DNA damage, or abnormal cellular proliferation (e.g., radiation exposure, hypoxia, and infection). This increase in p53 corresponds to arrest in the G1 phase of the cell cycle, thereby allowing for repair of DNA damage or progression to apoptosis. In HPV infected cells, E6 binds to p53 and causes degradation of the protein through a ubiquitin-dependent pathway. Thus less apoptosis and growth-arrest occurs, leading to cellular proliferation (34).
E6 has also been shown to interact with other proteins that are involved in a variety of cellular functions. E6 binds to transcriptional co-activators CBP/p300, further downregulating p53-mediated transcription (35). E6 also interacts with the PDZ proteins, which are involved in cell signaling and cell-to-cell adhesions, which ultimately affects lifecycle and carcinogenesis (36–38).
In addition to its inhibition of apoptosis through p53 interaction, E6 also upregulates the cellular telomerase complex. By synthesizing new DNA, telomerase maintains the length of the telomeric DNA at each end of a chromosome. Without this, the telomeres shorten with each cell division until they reach a critically short length and can no longer replicate. Therefore, by up-regulating the cellular telomerase complex, E6 further immortalizes the host cell (39,40). E6 expression is a late event in malignant transformation.
HPV E1/E2
Both E1 and E2 are proteins necessary for HPV DNA replication. E1 first initiates viral replication by binding near the start site of transcription. E1 complexes with the E2 protein, then binds to the viral genome, which initiates helicase activity (41). E1 also binds various other nuclear proteins for further replication of the viral DNA (42–44).
E2 is involved with DNA replication as well as genome transcription. In addition to forming a complex with E1 to assist in replication, E2 also works on the upper regulatory region to affect transcription of early genes (45,46). High concentrations of E2 repress transcription and low levels activate transcription. E2 therefore regulates E6 and E7 and loss of E2 expression can lead to carcinoma due to the unrestricted effects of E6 and E7 (47). Like E1, other interactions have been noted between E2 and various proteins involved with transcription as well as mitosis and cell division. E1 and E2 expressions are early events in the natural history of cervical dysplasia.
Viral Integration and Transformation to Malignancy
HPV genomes infect the cell via circular extra-chromosomal copies; however, over time, its viral genome can become inserted into host cell DNA, a process called integration. In low-grade cervical intraepithelial neoplasia (CIN), the HPV DNA is maintained in its closed, circular, episomal shape. However, in most high-grade CIN and cancers, the HPV DNA becomes integrated in the host chromosomal DNA (47–50). Many believe this is a necessary event to cervical carcinogenesis (51). Integrated HPV has been found to be present in 83% of invasive cervical cancers, as compared to 8% of low-grade CIN, suggesting that integration is highly associated with the transition of low-grade to high-grade lesions (52,53). Integration occurs at random sites in the host DNA; however, breaks in the HPV viral episome seem to occur at regions of genomic instability, such as fragile sites. Often the E2 ORF is disrupted during these breaks (54). As noted previously, E2 concentration affects the transcription of E6 an E7. Thus, once integration has occurred, disruptions in the E2 ORF lead to increased production of E6 and E7, thereby promoting immortalization and the oncogenic potential of the cell.
SECTION 2: EPIDEMIOLOGY OF HPV INFECTIONS
Prevalence
HPV is the most common of all sexually transmitted infections; while age, race, geographic region, and other modifiable risk factors also correlate with new infections, age, and sexual behaviors are most clearly linked to new HPV infections. Early studies likely underestimated the prevalence of HPV infections. As newer molecular technologies have been developed, detection of viral infection improved, even at low levels (55). In addition to variability surrounding detection methods, HPV positivity may be episodic, corresponding to times of viral shedding.
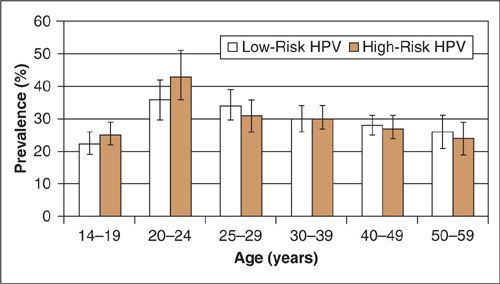
Utilizing polymerase chain reactions (PCR) on self-collected cervicovaginal specimens from over 4,000 US females, ages 14 to 49, the prevalence of HPV infection was recently estimated as part of the 2011 National Health and Nutrition Examination Survey (NHANES) (1) (Fig. 7.4). The overall prevalence of the 37 separate types was 42.5%. Prevalence was lower among females aged 14 to 19 (32.9%) and highest among females 20 to 24 years (53.8%), which is the peak age of new exposures. In that study, prevalence varied by race with non-Hispanic Blacks having the highest prevalence (59.2%) followed by Mexican Americans (44.2%) and non-Hispanic Whites (39.2%). HPV positivity was significantly associated with poverty, sexual activity (including number of partners, age, and first encounter) and history of genital warts.
HPV Prevalence Worldwide
Worldwide age-related trends are similar to US cohorts (56). Women less than 25 years old showed the highest prevalence of HPV infection, and in most regions of the world showed an overall decrease in prevalence with increase in age. In Africa, prevalence ranges from 12% to 55%, though most trials consisted of younger cohorts of women. In Central and South America, prevalence has been reported to be as high as 64%, and interestingly many studies showed an overall decrease in prevalence with age followed by an upward trend in women over 50. Canadian prevalence was lower than in the US, with a peak incidence of 25%. Chinese trials showed a range of 6% to 53% while Japanese trials generally reported lower prevalence of less than 15%. Studies of Indian women show ranges from 0% to 45%. European prevalence was almost consistently lower than in the United States with a peak of approximately 20% in young women (56).
FIGURE 7.4. U.S. Prevalence of HPV infections in Women.
Source: Reprinted with permission from Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis 2011;204:566–573.
HPV Prevalence in Men
The trends in prevalence and clearance in men are inextricably linked to the epidemiology of HPV infections in women. HPV testing in men has limited yield when compared to HPV testing in women, due to the generally keratinized surfaces where HPV-infection is present in men. The largest US population–based trial investigating HPV infection in men, where sampling was thoroughly performed, demonstrated a prevalence of 61% of any type of HPV infection. Specifically, high-risk HPV infections were found in 23%, with HPV 16 (7%) and HPV 51 (6%) being the most common (57). Additional data from the NHANES survey showed that men have a higher incidence of oral HPV infection of 10% versus 4% (58). In contrast to trends in women, prevalence of any HPV infection was not affected by age. Only low-risk HPV infections increased with age. Clearance of HPV infections seems to occur quicker in men, with an average clearance time of any HPV infection of approximately 6 months. In one study, nearly 75% of HPV infections in men were cleared within 1 year (57). Male circumcision significantly reduces genital HPV prevalence in men, which has led some to suggest circumcision as a method to reduce HPV-related disease burden in endemic communities where vaccination and screening is not yet feasible (59). In contrast to squamous cell carcinomas (SCC) of the female genital tract, HPV has only been detected in half of invasive penile SCC (60).
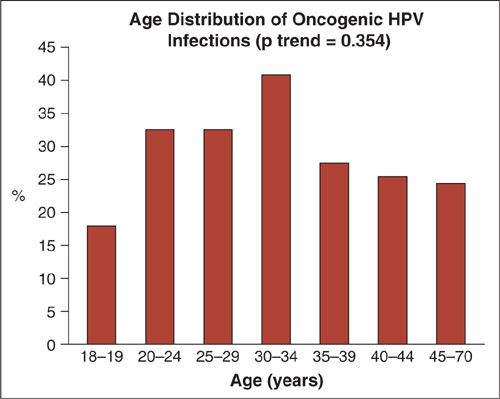
FIGURE 7.5. HPV prevalence in US Men.
Source: Reprinted with permission from Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008;17:2036–2043.
Natural History of HPV Infection (Rates of Abnormal Cytology, CIN, Cancer)
Despite its high prevalence, the majority of HPV infections are cleared by the body’s immune system. Only a minority of persistent high-risk HPV infections result in CIN and an even smaller subset progress to invasive cancer.
Determining the exact rate of HPV clearance is complex. Some trials examine existing infections (prevalence trials) while others follow women who develop new infections (incident trials). Even within incidence-based trials, methods vary and any subject may have a combination of low-risk, high-risk, preexisting, or incident HPV infections. It is important to also note that though a negative HPV test likely represents clearance of infection, it may also represent a “latent,” subclinical infection. This concept is derived from a number of sources including data examining HIV positive women who became HPV positive in the absence of new sexual exposures, suggesting a reactivation of viral shedding during periods of immunodeficiency (61). Also, in long-term cohort studies, recurrent infections are often HPV types that the patient has previously had, suggesting reactivation (62). However, once cleared, very few HPV type-specific infections reappear and far fewer lead to clinically relevant disease such as high-grade CIN or cervical cancer (63).
The 1-year clearance rate of incident HPV infection ranges from 40% to 70%. Two- to 5-clearance rates are as high as 70% to 100% in young women (10, 64–67). This rate may be significantly lower in older women; one trial showed no clearance of high-risk HPV infections in women over the age of 70 (67). Young women are more likely to clear infections than older women, and low-risk HPV infections clear more quickly than high-risk HPV infections (64). The longest course of persistent infection was seen with HPV types 16, 31, 54, and 53 (64,68).
The majority of women who acquire HPV infections do not develop CIN or invasive cancer. Of women who have persistent high-risk HPV infections, trials report variable rates of progression to CIN 2/3 from 8% to 28% (69–71). In cross-sectional studies, an estimated 3% to 5% will eventually develop cervical cancer without any intervention (72,73). In a prospective cohort study of New Zealand women with carcinoma in situ followed over decades, the cumulative incidence of cancer of the cervix or vaginal vault was over 30% (9).
Transmission
HPV infections are almost exclusively acquired during sexual exposure. Areas of microtrauma within the skin and mucosal surfaces are the likely sites of initial infection. Many natural history studies of HPV negative women at baseline who initiated intercourse confirm precedent sexual activity with new HPV infections (10). Furthermore, the rate of acquisition correlates with increasing number of sexual partners (74). Data regarding concordance and HPV transmission efficacy among couples are conflicting. In trials of young women and their male partners, HPV concordance ranged from 40% to 60%. Specific mechanisms that determine the efficiency of HPV transmission and concordance are poorly understood although it appears that length of sexual activity may increase concordance (75–77). Additionally, viral shedding may occur based on asynchronous replicative cycles of the HPV virus; thus, couples may appear to be less concordant than they really are at any specific time (68). In addition to cervical, vaginal, and penile detection, HPV has been detected on areas of unprotected genital skin such as the vulva and scrotum, providing an explanation as to why condoms offer some but not complete protection against HPV infection (78).
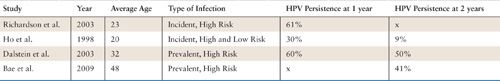
HPV Persistence |
Sources: Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003;12:485–490. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423–428. Dalstein V, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer 2003;106:396–403. Bae J, Seo SS, Park YS, et al. Natural history of persistent high-risk human papillomavirus infections in Korean women. Gynecol Oncol 2009;115:75–80.
SECTION 3: ADDITIONAL RISK FACTORS FOR DEVELOPMENT OF PREINVASIVE LESIONS OF THE LOWER GENITAL TRACT
Although HPV has been implicated as the most important and necessary risk factor for development of preinvasive lesions of the lower genital tract, other risk factors have been identified that either increase the risk of HPV infection or potentiate the progression of HPV infection to malignant transformation.
Sexual History
Even before data convincingly linked HPV to the development of cervical cancer, it was well recognized that various aspects of a patient’s sexual history put women at increased risk for developing preinvasive and subsequent invasive lesions of the cervix, vagina, and vulva.
Sexual history and behaviors strongly correlates with risk of acquiring HPV infection and developing preinvasive lesions (66). Overall, the number of recent and total lifetime male partners increases the rate of HPV infection, particularly high-risk HPV infection (66,79–82). The most recent data from the U.S. Centers for Disease Control (CDC) data show that number of lifetime partners is an independent risk factor for all races except non-Hispanic Black women, where no independent correlation is seen (83).
Early onset of sexual activity has also been shown as an independent risk factor for HPV infection in some studies (81,83). Some studies suggest that frequency of intercourse with one’s partner may also play a role (10). Characteristics of the partner, including number of lifetime partners (79) and multiple coincidental partners, have all been implicated in risk of HPV acquisition (10). The rate of having new partners and having known a new partner for shorter periods of time before having vaginal intercourse are also associated with increased risk of HPV infection (66,79).
Additionally, co-infection with other sexually transmitted diseases and vaginal infections has been associated with increased susceptibility to HPV infection. Both bacterial vaginosis and trichomoniasis are associated with HPV infection although the correlation is not as strong with development of CIN (84,85). A history of herpes simplex virus infection and vulvar warts also increase the incidence HPV infection (66). A history of previous chlamydial infection has long been implicated in the development of invasive squamous cell carcinoma of the cervix (86–88). Hypotheses regarding pathogenesis include induction of squamous metaplasia at the transformation zone, increase in microabrasions, as well as interference with immune surveillance (88,89). More recent cohort studies have found that the role of chlamydia in carcinogenesis is likely related to both in promoting the acquisition and persistence of HPV infections (90–92). Chlamydia has not been implicated in the development of adenocarcinoma in situ (AIS) or invasive adenocarcinoma (88).
Tobacco
In 2004, the IARC added cervical cancer to the list of cancers causally related to smoking (93) based on analysis of numerous trials in the prior decade. As more epidemiologic and natural history trials were performed, conflicting data emerged on the association between cigarette smoking and HPV acquisition, persistence, and progression. Confounding variables have also been a factor; women who smoke are less likely to be compliant with screening guidelines (94) and more likely to have more social stressors (95).
Most studies agree, though, that current smoking increases the prevalence of and persistence of HPV infection, particularly those that have a high-risk HPV type (96–99). Additionally, most trials suggested delayed clearance of HPV infection in women who smoke (100,101). Compared to nonsmokers and former smokers, current smokers have higher high-risk HPV viral loads (102).
The effects of smoking on the development of lower genital tract neoplasia are multifactorial. Nicotine and other byproducts of cigarette smoke have been readily identified in the cervical mucus of smokers (103). Benzo[a]pyrine, a known human carcinogen, has been found in cervical mucus and has been specifically noted to potentiate the HPV 16 and HPV 18 viral lifecycle (104). It has long been known that smoking causes a local immunological effect whereby the function of Langerhans’ cells are significantly decreased, thus affecting the innate immune system (105). Studies of women with preinvasive and invasive disease have shown that compared to nonsmokers, smokers show aberrant methylation of p16, a tumor suppressor gene (106). Other recently identified tumor markers in smokers with CIN include overexpression of cyclooxygenase-2 and Ki-67 and underexpression of p53, interleukin-10, and fragile histidine triad (107).
Oral Contraceptive Pills
The association between oral contraceptive pills (OCPs) on preinvasive and invasive diseases of the lower genital tract has been conflicting. The largest population-based study, which pooled data from trials involving over 50,000 women worldwide, did show an increased risk of preinvasive and invasive disease in women who were current users of OCPs, although this risk was eliminated when OCP use was discontinued (108). This study is criticized for its heterogeneity between trials and the possible confounding effects of sexual behavior in women on OCPs—thus, many have questioned the true association. Other large trials since then have shown that OCP use is not an independent risk factor for development of abnormal cytology, CIN, AIS, or invasive disease (109–111).
HIV and Immunosuppression
HIV infection is strongly associated with HPV infection. Additionally, HIV coinfection in one partner impacts the prevalence of HPV infection in the other partner (112). Higher incidence and prevalence as well as prolonged persistence of HPV have all been associated with women who are HIV positive (113). Rates of CIN are also higher among HIV positive women, independent of other risk factors (114). Additionally, CD4 count and HIV RNA levels correlate with high-risk HPV positivity (61). Although highly active antiretroviral therapy (HAART) has not been shown to definitively affect the incidence or persistence of HPV infections, it has been associated with better CIN outcomes and improves overall life expectancy of HIV positive women (115).
In addition to its effect on prevalence of HPV infection, other mechanisms related to coinfection of HPV and HIV have been proposed. Primarily, it is noted that a functional immune system is required to keep HPV in a latent and subclinical state. Thus HIV infection, in addition to other forms of immunosuppression, predisposes to progression and reactivation of HPV infection (61). Additionally, different molecular pathways have been proposed for HIV-related preinvasive lesions including a higher frequency of microsatellite instability in HIV associated CIN (116). Based on patterns of local pro-inflammatory immune markers, it appears that persistent HPV infection might make someone more susceptible to HIV infection as well.
Another group of women known to be at high risk for preinvasive HPV-associated lesions are transplant recipients and other patients on chronic immunosuppressive therapy such as those with Systemic Lupus Erythematosus. Small, early studies noted a higher incidence of HPV infections and up to 16 times the rate of invasive cervical cancer in women with a history of renal transplant compared with controls (117). However, these data are limited by less effective methods of HPV testing and lack of control for covariates. In a recent prospective 10-year trial of 48 women with renal transplants, no increased risk of HPV infection, high-grade cytology, or preinvasive lesions was observed (118). A large Swedish cohort study found that the rate of vulvar malignancy in women who had undergone solid organ transplant was 26 times higher than the general population. Additionally, the rate of vaginal cancer was 16 times higher. Rates of cervical cancer were not statistically higher and preinvasive lesions were only marginally increased (119). A U.S. cohort of transplant recipients was analyzed and only vulvar carcinoma demonstrated a statistically significant higher incidence in transplant recipients. In fact, the incidence of vulvar carcinoma following a solid organ transplant was higher than the incidence of cervical cancer (7.5 vs. 5.8 per 100,000 person years) (120). This may reflect improved cervical cancer surveillance in this population. However, it may also reflect the different pathology of vulvar lesions in immunocompromised women. Smaller studies suggest rates of 90% to 100% HPV positivity in preinvasive and invasive vulvar lesions in women with transplants (121,122).
SECTION 4: PATHOLOGY OF PREINVASIVE LESIONS
Cervix
Formerly known as cervical dysplasia and carcinoma in situ (CIS), preinvasive squamous cell lesions of the cervix are now categorized as various levels of CIN. More specifically: mild, moderate, and severe dysplasia/CIS are now termed CIN 1, CIN 2, and CIN 3, respectively.
CIN Localization
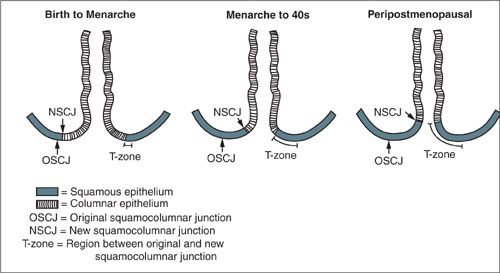
The vast majority of CIN develops in the transformation zone of the cervix, located at the squamo-columnar junction between the columnar epithelium of the endocervix and the squamous epithelium of the ectocervix. This area has been so-called “transformed” due to the process of metaplasia (Fig. 7.6). Prior to menarche, the transformation zone does not exist. The squamo-columnar junction occurs exactly at the level of the external cervical os. During menarche (as well as during pregnancy), the columnar epithelium of the endocervix appears on the ecto-cervix and is termed an “ectropion” or “cervical ectopy.” This columnar epithelium gradually becomes replaced by stratified squamous epithelium due to a variety of factors including alterations in pH and other hormonal changes. This area of former columnar, now squamous, epithelium that lies between the original squamocolumnar junction and the new squamocolumnar junction is termed the transformation zone. The transformation zone is the primary site for inducing cell-mediated immunity in the lower genital tract, and its role in the acquisition of not only HPV infections, but also HIV infections is still being elucidated (123,124).
Microscopic Appearance of CIN
Nuclear atypia is a prominent feature of CIN, which can occur at any level of dysplastic severity. It occurs as a result of enhanced proliferation, replication, and intracellular assembly of viral particles in HPV infected cells. The nuclei are hyperchromatic and frequently multinucleated; additionally, the nuclear outline is often irregular rather than round. The swollen appearance of the cells, often termed as koilocytes, is a result of viral particles present in the episomal area of the cell.
In addition to nuclear atypia, CIN 1 to 3 histology shows aberrant cytoplasmic differentiation. The basaloid cells, which are normally found as a single layer in contact with the basement membrane, replace the normal epithelium. These cells display nuclear crowding, loss of normal cell polarity, pleomorphism, and abnormal mitotic figures. The extent of these abnormal basaloid cells determines the grade of CIN. If the undifferentiated basaloid cells involve the lower third of the epithelium, the lesion is CIN 1; if these cells are present two-thirds of the way through the epithelium, the lesion is CIN 2; and if the extent is greater than two-thirds, the lesion is termed CIN 3. In order to better classify the degree of CIN, molecular markers can be used by pathologists. For instance, p16INK4a is a validated marker for biologically relevant CIN (125).
FIGURE 7.6. Impact of age on the location of the squamocolumnar junction. As females age, the location of the squamocolumnar junction on the cervix moves. The movement of the squamocolumnar junction defines the transformation zone.
This classification system was developed when all of CIN was thought to be a spectrum of disease. Now we understand that CIN 1 usually represents a benign process that is merely a cytomorphologic representation of an active HPV infection; whereas higher-grade lesions, especially CIN 3, are truly premalignant. CIN 2 is a poorly reproducible category, with high levels of inter-observer variability (126). In one trial, agreement between pathologists was as low as 13% in the category of CIN 2 compared with 81% agreement with CIN 3 lesions (127). CIN 2 is now considered an equivocal diagnosis because it can include either benign HPV effects or precancerous lesions (125). In an analysis of a vaccine trial, agreement between biopsy and LEEP specimen improved when CIN 2 and CIN 3/AIS were grouped as a single predictive measure of high-grade disease (128). Due to limitations surrounding reproducibility, many institutions characterize lesions as either CIN 1 or CIN 2/3 (also known as CIN 2+); and often include additional molecular markers such as p16INK4a and other proliferation markers to improve clinically relevant disease ascertainment (Fig. 7.7).
In CIN 1, the lower third of the epithelium displays nuclear atypia of immature basaloid cells while the remainder of the epithelium often shows the additional cytopathic effects, termed koilocytosis, which are directly related to an active HPV infection. Koilocytes are cells with atypical nuclei and perinuclear clearing, also known as vacuolization; they can be seen in both CIN 1 and CIN 2/3, although it is more common in CIN 1. The large, perinuclear vacuole is often a result of activation of viral E5 and E6 proteins (129).
Natural History of CIN
CIN 1 is the most common histologic diagnosis after colposcopic biopsy. Fortunately, very few of these lesions actually progress to a higher grade of CIN. Most will remain persistent or regress spontaneously as the HPV infection is cleared (130). In both prospective and retrospective trials, incidence of CIN 2/3 18 to 24 months after diagnosis of CIN 1 ranges from 4% to 10% (131,132). A retrospective analysis of the ASCUS-LSIL Triage Study (ALTS) (133) found that a diagnosis of CIN 1 on pathology (compared to a negative biopsy, or no biopsy taken) was not a risk factor for developing CIN 3. Therefore, CIN 1 is considered a nonneoplastic lesion and is not a disease that should require any extirpative treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree