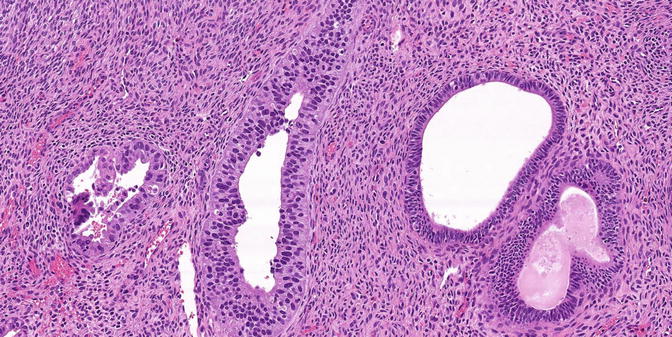
Fig. 8.1
Serous EIC in a glandular pattern and flat pattern
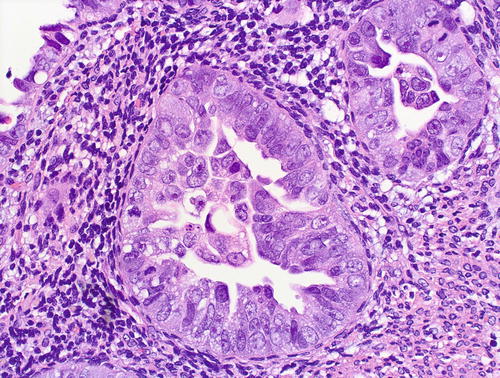
Fig. 8.2
Serous EIC, glandular pattern: cytologic features – a given case may variably show severe pleomorphism, hobnail nuclei, hyperchromasia with coarse chromatin pattern, nucleolomegaly, intraglandular budding, eosinophilic cytoplasm, and abundant mitotic figures
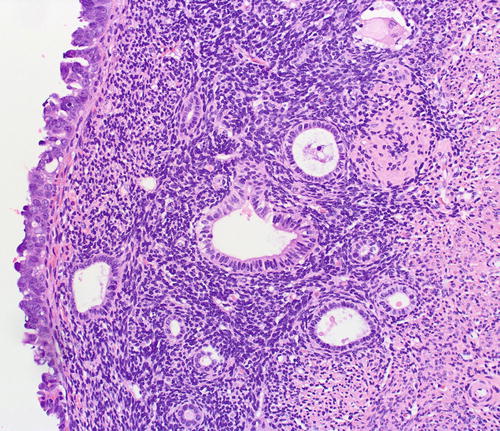
Fig. 8.3
Serous EIC in a flat pattern, left field
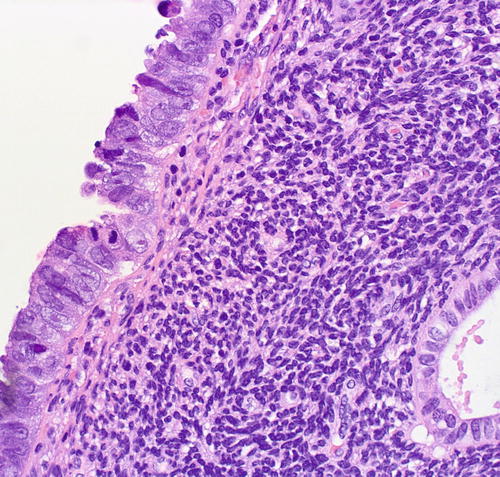
Fig. 8.4
Serous EIC in a flat pattern, cytologic features
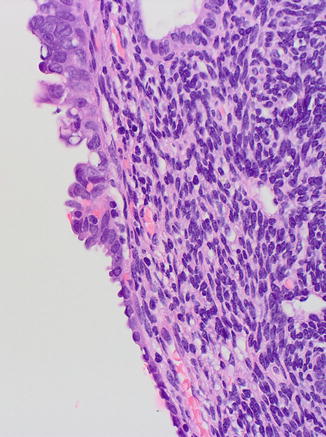
Fig. 8.5
Serous EIC (upper field) showing transition to EmGD (midfield) and to resting endometrium (lower field)
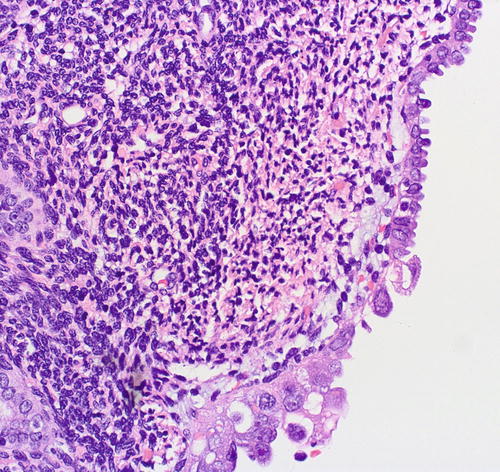
Fig. 8.6
Serous EIC (lower field) showing abrupt transition to resting endometrium (upper field)
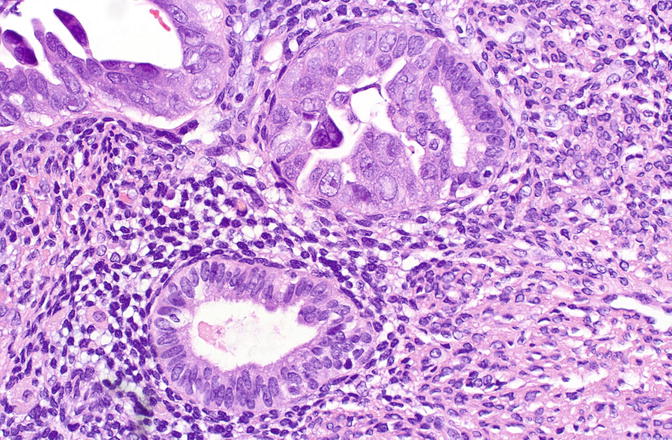
Fig. 8.7
Serous EIC showing abrupt transition to nonneoplastic cells within a single gland (upper field)
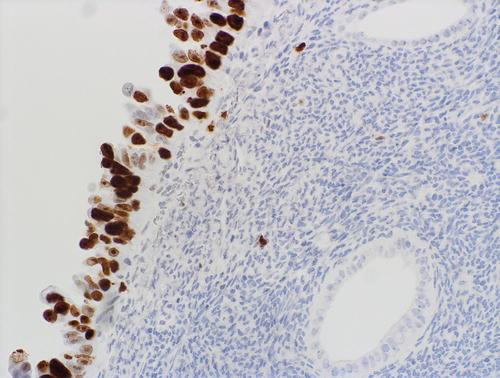
Fig. 8.8
Serous EIC showing aberrant pattern of p53 expression
The histologic differential diagnosis includes papillary syncytial metaplasia (PSM) with degenerative atypia [56–58], radiation-associated atypia, or metastases. The overlap between PSM and serous EIC is related to potentially significant atypia and increased expression of p16 and p53, concomitant with decreased expression of hormone receptors in the former [56–58] (Figs. 8.9 and 8.10). However, unlike serous EIC, PSM lacks mitotic figures, has a low proliferative index, lacks expression of HMGA2, and has a wild-type pattern of p53 staining [56–58]. Radiation-associated atypia is usually more diffuse, lacks mitotic figures and has a low proliferative index, lacks a serous carcinoma-like immunophenotype, and does not display the intraglandular budding that is typical of serous EIC (Figs. 8.11 and 8.12). Endocervical adenocarcinoma in situ may also present as an intraepithelial lesion in an endometrial polyp or in background endometrium [63]. As detailed below, adnexal serous carcinomas may also present as minute or focal lesions in endometrial samples [64].
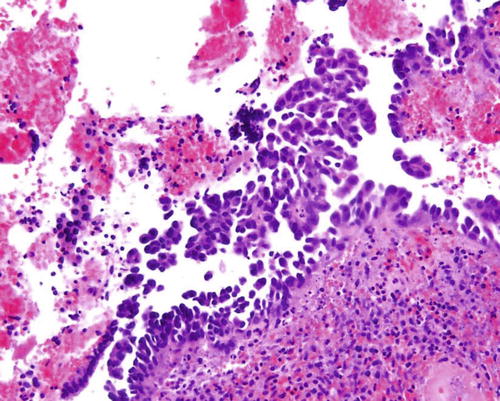
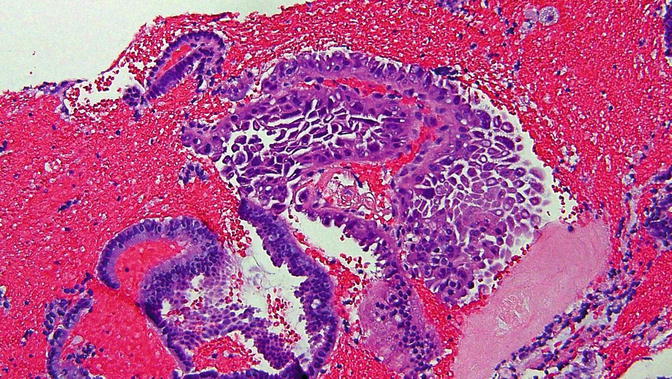
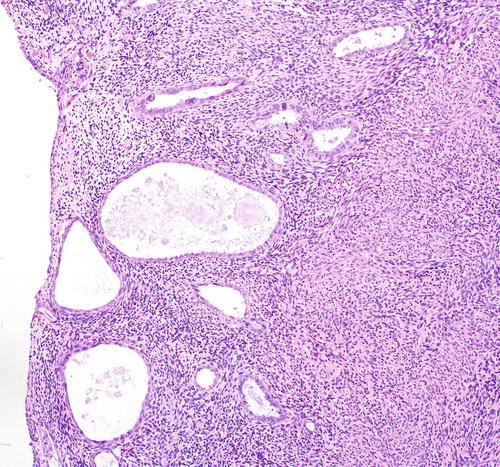
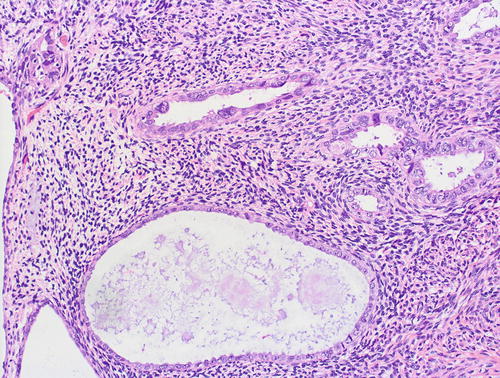

Fig. 8.9
Papillary syncytial metaplasia, a lesion in the differential diagnosis with serous EIC

Fig. 8.10
Papillary syncytial metaplasia , a lesion in the differential diagnosis with serous EIC

Fig. 8.11
Radiation-associated glandular atypia in the endometrium, a finding that is in the differential diagnosis with serous EIC

Fig. 8.12
Radiation-associated glandular atypia in the endometrium: Higher magnification of Figure 8.11
Serous EIC, Extrauterine Extension, and Pelvic Serous Carcinogenesis
The notion that non-myoinvasive or superficially invasive ESC may potentially show extrauterine extension had long been recognized, even from some of the earliest descriptions of ESC (1). Serous EIC has been identified in 58–89 % of ESC [28, 35]. Although EIC is rarely identified without concomitant ESC, analy ses of small groups of patients have conclusively established that EIC may be associated with peritoneal carcinomatosis in the absence of any other serous neoplasm in the uterus [36, 37]. The frequency with which this occurs is not entirely clear. However, for the larger group of lesions defined as minimal or only very superficially invasive, up to two-thirds of cases may be associated with extrauterine extension [16]. Morphologically, the extrauterine deposits display the same features as the endometrial lesions [38] and are usually less than 2 cm each [39]. Similarly, both intrauterine and extrauterine lesions have similar immunophenotypes regarding estrogen receptor, progestin receptor, p53, WT1, and Ki-67 expression [38]. At the molecular level, both uterine and extrauterine lesions within a given patient have been shown to display identical TP53 mutations [39, 40]. However, the interrelationships between these lesions may be more complicated.
A very small subset of serous carcinomas in the endometrium may actually represent either drop metastases from the adnexa that manifest in the endometrium with an EIC-like intraepithelial growth pattern. Many of such lesions are associated with extrauterine serous carcinomas that are independent primaries as evidenced by the concomitant presence of corresponding precancerous lesions at the extrauterine site. It is known that some cases of EIC may be associated with serous tubal intraepithelial carcinoma (STIC ) in the fallopian tube. In one study of 22 consecutive cases of serous carcinomas involving the endometrium [41], the fallopian tubes were submitted according to the SEE-FIM protocol [42]. 50 % of cases showed adnexal involvement, including five wherein a STIC was identifiable. In all five cases, the endometrial lesions tended to be noninvasive or only minimally invasive of the myometrium , and identical TP53 mutations were shared by both tubal and endometrial lesions in a subset of cases [41]. In another study 8 % of ESC showed STIC when the fallopian tube was processed using the SEE-FIM protocol [35]. Extensive sectioning of the endometrium in patients with adnexal serous carcinomas has identified serous EIC in about 15 % of cases [43]. In our own recent analysis of samples from 21 patients with serous EIC, cellular lineage relationships between intrauterine and extrauterine lesions were assessed by TP53 mutation analysis. Based on the patterns of concordant or discordant mutation for this gene, we concluded that the extrauterine disease associated with serous EIC may be from the endometrium (47.6 % of cases), adnexa (23.8 %), or both (28.6 %) [39]. All of the aforementioned findings, in total, suggest either that some cases of ESC (EIC or otherwise) are of extrauterine origin, that the primary lesion accordingly contributes to the extrauterine disease burden, or that both the intrauterine and extrauterine lesions are independent, being simply reflective of an increased propensity of serous carcinogenesis that is generalized in the upper genital tract.
Serous EIC as a Precursor Lesion
The conclusion by Ambros et al. in 1995 that serous EIC “is the likely precursor” for uterine tumors displaying serous differentiation was based on their intraepithelial growth pattern as well as their statistically significant, nearly exclusive association with carcinomas with a serous component [30]. Subsequently published studies have shown that serous EIC and ESC display broadly similar phenotypic patterns, including a comparable frequency of expression of proteins such as p53, p16, IMP3, HMGA2, Nrf2, hormone receptors, Cyclin E1, and HER2/neu [44–51]. This is not unexpected, since serous EIC is definitionally composed of cells that are identical to ESC.
At the molecular level, serous EIC and ESC have been shown to be very similar and to share a broad molecular profile. Kuhn et al. [52] performed a comparative mutational analysis in samples from nine patients with serous EIC and ESC pairs. All nine pairs showed concordant PIK3CA, PPP2R1A, and TP53 mutational profile, whereas eight of the nine pairs had concordant FBXW7 mutation status between these two components. The single discordant pair contained a FBXW7 p.Asp440Asn mutation that was absent in serous EIC but present in the associated ESC [52]. FBXW7 mutation may be associated with Cyclin E1 overexpression at the protein level, as can CCNE1 amplification, the underlying gene for the Cyclin E1 protein. It has subsequently been shown in another paired analysis that 45 % of ESC and 41 % of serous EIC showed CCNE1 amplification as assessed by fluorescent in situ hybri dization and that there was generally a high concordance in CCNE1 copy numbers between the pairs [53]. However, there is evidence that although serous EIC and ESC are similar, they are not identical, and this may lend credence to the postulation that serous EIC precedes ESC. The frequency of TP53 mutations is generally higher in ESC than in serous EIC (72–78 % in serous EIC vs. 90–96 % in ESC) [24, 54]. Loss of the wild-type p53 allele, as inferred from loss of heterozygosity at chromosome 17p, has been observed in 100 % of ESC and in 43 % of serous EIC [54]. Occasional cases of serous EIC and their associated ESC display discordant TP53 mutations (7 % of cases in our analysis [24]). Additionally, as previously indicated, FBXW7 mutations in the same patient are also occasionally discordant between these lesions. Also supporting the concept that serous EIC precedes ESC is the morphologic observation of their growth pattern and size. It is logical that a small, highly proliferative lesion with an intraepithelial growth pattern will eventually become a large lesion with an invasive growth pattern. Accordingly, serous EIC is placed before ESC in our model and is its likely precursor [16, 17]. However, this placement of serous EIC is merely a conceptual component of the framework by which endometrial serous carcinogenesis can be studied and understood. For practical and clinical purposes, this distinction is irrelevant, since the evidence indicates that serous EIC is already a cancer with significant potential for metastasis [36, 37], rather than a true precancer that is associated with an increased risk of cancer but which is in and of itself not malignant [55]. Serous EIC, when identified alone, is therefore best conceptualized as ESC at an early patholog ic phase and/or with an unusual intraepithelial growth pattern, and patients with serous EIC should be managed in the same manner as their counterparts with the conventionally invasive neoplasm [13, 32].
Endometrial Glandular Dysplasia
Studies published during the past decade have established endometrial glandular dysplasia (EmGD) as the most likely precancerous lesion for ESC [21–26, 59]. EmGD was originally described in 2004 [21], following works predicated on the hypothesis that there is a morphologically identifiable lesion that precedes serous EIC and which is precancerous. In the seminal reports that described the entity, the endometrium adjacent to a cohort of serous EIC, ESC, and endometrioid carcinoma cases was evaluated in detail. Atypical glandular lesions were identified 53 % of the ESC cases and 1.7 % of the endometrioid carcinomas [21]. These lesions were then studied and form the basis of the EmGD entity. The average age of the patients with an EmGD lesion was 65 years (range 57–79 years) [21].
Morphology, Immunophenotype, and Differential Diagnosis
EmGD are microscopic lesions, and it is rare for any single focus to exceed 2 mm. However, we have occasionally encountered them extensively involving an endometrial polyp. By definition, EmGD shows a level of atypicality that renders them distinct from the background endometrium, but which is not as severe as is characteristic of serous EIC (Figs. 8.13, 8.14, 8.15, 8.16, 8.17, 8.18, and 8.19). This atypicality is manifested in oval to round nuclei that are typically two- to threefold enlarged as compared with the background resting endometrium (in which about 80 % of them are seen [21]). This is as compared with serous EIC cells, which are usually 4–5 times larger. EmGD may be in the form of single or clustered glands or in overlying flat epithelium. EmGD is frequently multifocal. Nucleoli may be seen but are not conspicuous. The chromatin pattern is variable. Mitotic figures and apoptotic bodies are not readily apparent [21]. Transitional areas between EmGD and serous EIC are frequently observed [21].
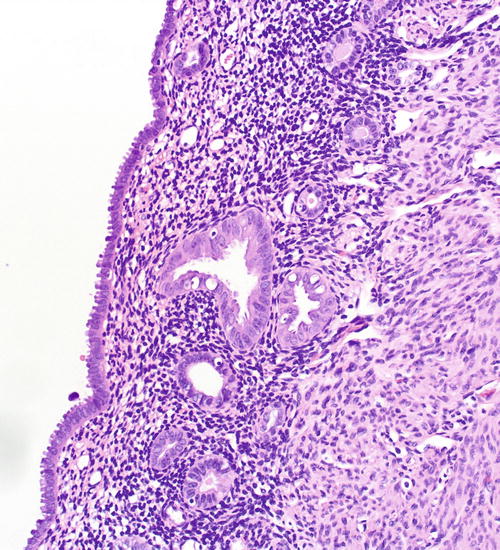
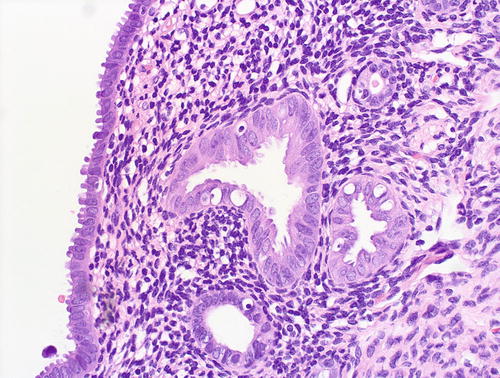
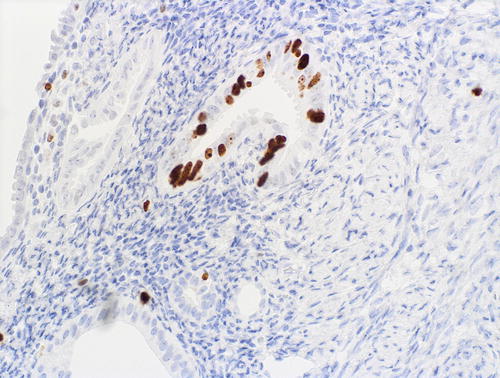
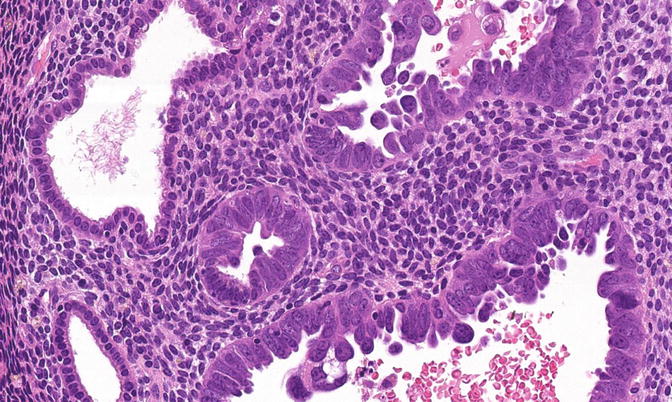
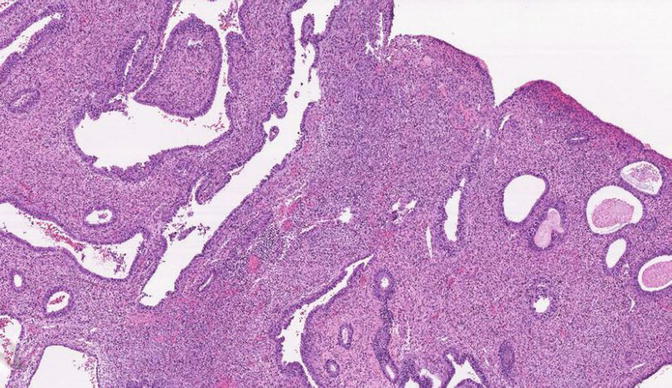
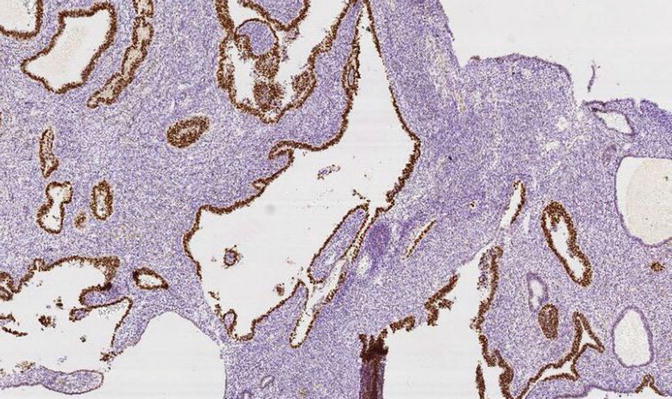

Fig. 8.13
A focus of EmGD, represented here as a pair of glands (center field) showing notably increased atypia above the background and overlying epithelia

Fig. 8.14
EmGD: higher magnification of glands shown in Fig 8.13

Fig. 8.15
A focus of EmGD showing increased proliferative activity relative to background epithelia, as assessed with MIB1 immunohistochemistry

Fig. 8.16
EmGD (middle gland) compared with background endometrial glands (left field) and serous EIC (right field)

Fig. 8.17
A rare case of EmGD diffusely involving an endometrial polyp

Fig. 8.18
A rare case of diffuse EmGD invol ving an endometrial polyp. This case showed diffuse expression of the p53 protein
Most cases of EmGD are recognizable by paying careful attention to morphologic attributes. However, given that EmGD definitionally occupies a nebulous “intermediate” point between well-recognizable morphologies (resting endometrium and serous EIC), diagnostically problematic cases may be encountered. In these cases, which are most relevant in a biopsy or curettage, the differential considerations include a benign gland with reactive or metaplastic changes and EmGD. Immunohistochemical studies may be useful in these scenarios. IMP3 may highlight these foci relative to background endometrium, but IMP3 is relatively insensitive for this purpose [48]. Their MIB1 proliferative index is typically distinct from the background [21] (Figs. 8.13, 8.14, and 8.15). Thus, comparison with the background endometrium is important in assessing these foci, and if the background endometrium is not resting, MIB1 is less useful. p16 may be useful in delineating glands that are biologically abnormal but whose morphologic features are equivocal, since EmGD most frequently expresses p16 diffusely, whereas background glands show a “mosaic” pattern (Figs. 8.20 and 8.21). However, p16 must be utilized in conjunction with other markers, so that tubal metaplasias, which may be cytologically atypical and are usually p16 positive, are not mischaracterized as EmGD. In our original studies, p53 immunohistochemistry was also useful, since most of our EmGD cases had p53 staining scores that were intermediate between serous EIC and resting endometrium [21]. Based on current understanding of p53 staining patterns, only diffuse staining is acceptable as being indicative of an aberrant TP53 status. Distinct and significant p53 staining in a putative EmGD (i.e., morphologically atypical) gland relative to the background is consistent with EmGD. Problems associated with the use of p53 include the fact that only 43 % of EmGD lesions display a TP53 mutation [24] and the fact that a subset of TP53-mutated cases lack an intact protein and accordingly are p53 negative by immunohistochemistry. In general, EmGD foci also display reduction (relative to uninvolved glands) or loss of expression of the estrogen and progesterone receptors.
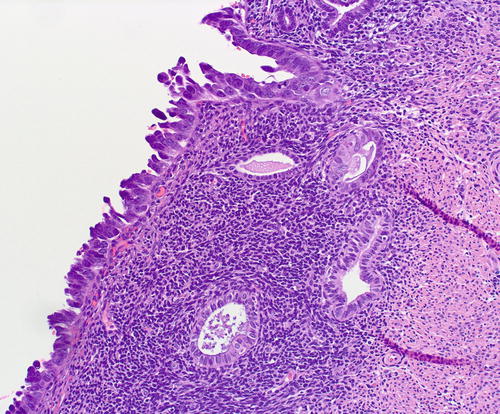
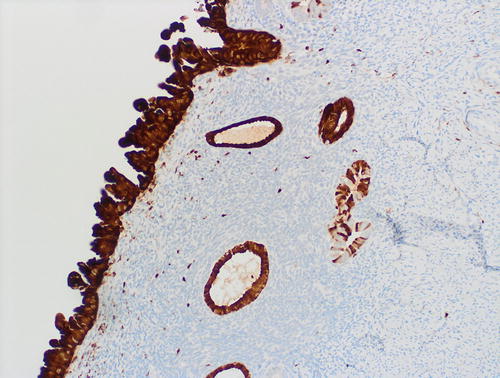

Fig. 8.20
Underlying the serous EIC (left field) are 2 subepithelial glands with mild atypia, which raises the diagnostic question of tubal metaplasia with degenerative nuclear changes versus EmGD

Fig. 8.21
Same case shown in figure 8.20, highlighting the limited utility of p16 immunohistochemistry in distinguishing tubal metaplasia with reactive changes from EmGD. p16 was diffusely expressed in the serous EIC, in some of subepithelial glands with tubal metaplasia, and in a “mosaic” pattern in the single subepithelial gland without tubal metaplasia (right field). p16 should always be used in conjunction with other markers in this context. In this case, the metaplastic glands showed no increase in proliferation as assessed by MIB1
EmGD as a Precancer
In 2004, the US National Cancer Institute sponsored a co nsensus conference on precancers, and participants defined five criteria that putative precancers must meet: (1) there is a method by which the precancer can be diagnosed; (2) it differs from the background normal tissue from which it originates; (3) the precancer shares some but not all molecular and phenotypic attributes with the cancer into which it develops; (4) there is evidence that when the precancer develops into cancer, the cancer arises from cells within the precancer; and (5) there is some evidence that the precancer is associated with an increased risk of cancer [55].
The available evidence suggests that EmGD is the most likely precancerous lesion to ESC. As indicated in the sections on morphology and immunohistochemistry, there are modalities by which EmGD can be diagnosed, the lesions are definitionally distinct from the background endometrium and most frequently have a phenotype that is distinct from it as well. As such they fulfill criteria 1 and 2. Since TP53 mutations appear to be one of the earliest molecular events in serous carcinogenesis, they offer a valuable framework for studying their putative precancers. Linkage analyses of the TP53 gene provide supportive evidence that EmGD is a precancer. The load of TP53 mutations tends to progressively increase from EmGD to serous EIC and ESC. In our study, the rates of TP53 mutations in resting endometrium, EmGD, serous EIC, and ESC were 0 %, 43 %, 72 %, and 96 % [24]. The differences bet ween EmGD and serous EIC or ESC were statistically significant [24]. Loss of heterozygosity at the chromosomal region for TP53 and 1p is observed in 31 % and 18 % of EmGD, respectively, but in 78 % and 47 % of cancerous areas within the same uteri [22]. When EmGD, serous EIC, and ESC are present in the same uterus, the specific TP53 mutations within these lesions are generally highly concordant [24]. Similar findings have been found by loss of heterozygosity analyses using microsatellite polymorphic DNA markers [22] or by HUMARA-based analyses [16]. These studies, especially the concordance of TP53 mutations between EmGD and ESC/serous EIC within the same uteri, provide compelling linkage evidence that the cancers in these uteri originated from the EmGD, and the differences in mutational load indicate that EmGD displays some but not all the properties of the well-developed malignancy. Then, the latter can also be intuited from morphologic differences and frequency of expression of markers such as IMP3 (which are significantly more frequently expressed in the cancers) [48]. In mice, deletion of the TP53 gene homologue Trp53 in an endometrium-specific fashion resulted in the development of a variety of type II endometrial malignancies. Analysis of the background, nonneoplastic endometrium in these mice identified a series of lesions whose features were diagnostic of EmGD and serous EIC [60]. Interestingly, in Trp53 knockout mice, the protein KPNA (karyopherin alpha 2), a mediator of nucleocytoplasmic transport [61], is expressed in ESC but at comparably low levels in EmGD or serous EIC and not at all in normal endometrium [62]. This not only reinforces the aforementioned point that differences exist between serous EIC and ESC and between EmGD and serous EIC and ESC but also that EmGD is distinct from the background endometrium.
The fact that only half of EmGD cases display TP53 mutations indicates that although this is an early molecular alteration, either (1) the half without TP53 mutations display cytologic atypia but are actually not neoplastic or (2) these lesions possess an undefined molecular alteration other than TP53 that renders them neoplastic and cytologically atypical and only acquire TP53 as a primary “driver” molecular event to malignant transformation later in their evolution, since TP53 is the most common molecular alteration in ESC [52]. It is also worthwhile to note that a different pathway of evolution may be operational for a subset of cases, since not all cases of ESC display TP53 mutation, and a variety of authors using a variety of different analytic approaches have consistently demonstrated that anywhere from 4 to 18 % of cases lack this mutation [24, 52, 54].
The final hallmark of a precancer is that there is some evidence that the precancer is associated with an increased risk of cancer [55]. The risk for malignancy posed by an isolated diagnosis of EmGD in an endometrial sampling has not been systematically evaluated in long-term prospective studies. It is important to note that such studies are difficult to construct even for the significantly more common precancers for Type I endometrial carcinomas, since it would likely entail a patient “observation only” approach after diagnosis. Additionally, the risk to life posed by failing to prevent the evolution of a lesion to ESC is likely to be significantly higher than to endometrioid carcinoma. The aforementioned EmGD-associated risk is presumed to be elevated based on a single retrospective study of endometrial biopsies and curettages that preceded the diagnoses of ESC in a large cohort [23]. We studied the endometrial samples that preceded (3 months or earlier) the hysterectomies for 250 ESC cases and a control group of 258 benign cases. 27 biopsy specimens from the ESC group and 29 samples from benign control group were ultimately assessed in detail. A total of ten EmGD cases were identified from both groups, 90 % of which were from the ESC group. The period between the EmGD-bearing biopsy and the diagnosis of ESC or serous EIC ranged from 16 to 98 months (average 33 months) [23].
All of the above findings justify the characterization of EmGD as a precancerous lesion that precedes serous EIC.
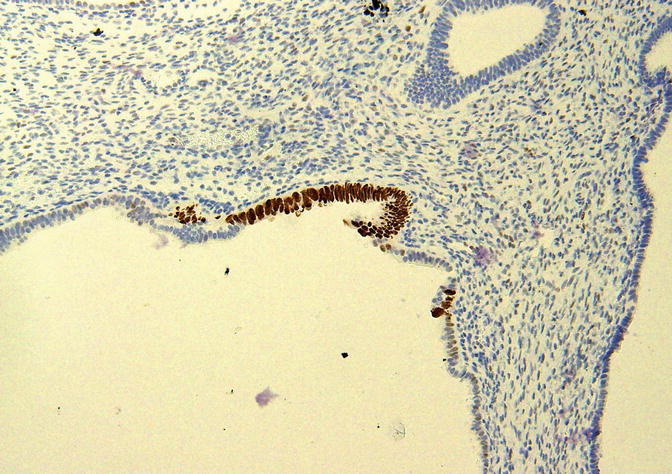
p53 Signatures
A p53 signature is a morphologically unremarkable segment of epithelial cells displaying moderate to strong immunoreactivity for the p53 protein as assessed by immunohistochemistry (Fig. 8.22). These foci were identified by immunohistochemical analyses of the background endometrium in carcinomas and have been proposed as the first step in endometrial serous carcinogenesis in our model [16, 17]. They are multifocal in at least 89 % of cases [18]. Zhang et al. identified p53 signatures in the endometrium associated with 39.1 % of ESC, 37 % of serous EIC, and 3.3 % of endometrioid carcinomas and in 1.7 % of benign endometrial samples [18]. Jarboe et al. identified p53 signatures in 70 % associated with 70 % of serous EIC and in 4 % of 137 endometrial polyps [19]; other authors have reported less well-defined increases in p53 expression in endometrial polyps [68, 69, 73]. Koi et al. [70] identified p53 signatures in 11 % of the endometrial tissues of 82 women with a variety of benign diseases. Nguyen et al. identified p53 signatures in the background endometrium associated with 24 % of ESC, 0 % of CCC, and 20 % of carcinosarcomas [20]. In the latter study, of the eight non-endometrioid tumors with p53 signatures, 88 % were associated with serous EIC [20]. The p53 signatures are occasionally observed in direct continuity with serous EIC [19]. These signatures display a low proliferative index (4–5 %) [19, 20] and express estrogen receptor-alpha [20, 70]. In our analysis of microdissected samples of p53 signatures, 42 % of cases showed at least one TP53 gene mutation [18]. A high degree of concordance was identified between the EmGD, serous EIC, and ESC lesions within a given uterus, as at least one identical TP53 gene mutation was found in all three lesions in 50 % of cases [18]. Similarly, Jarboe et al. [19] found identical TP53 mutations in paired p53 signatures and serous carcinomas in two (67 %) of three cases analyzed.