Postoperative Complications
Because cesarean section is the most commonly performed obstetric surgical procedure, most postoperative complications are encountered after this operation. Pelvic infection is most often encountered, but other less common conditions that cause excessive morbidity and mortality include aspiration pneumonia, deep venous thrombosis, and pulmonary embolism.
DIFFERENTIAL DIAGNOSIS OF FEVER
Most persistent fevers after childbirth are caused by genital tract infection. This especially is likely if extensive vaginal or uterine manipulation, prolonged membrane rupture, or intrauterine electronic monitoring attended the preceding labor. Regardless, every postpartum woman whose temperature rises to 38°C should be evaluated for extrapelvic causes of fever as well as for puerperal infection. Filker and Monif (1979) reported that only 21 percent of febrile women (first 24 hours) delivered vaginally were found to have infection in contrast to 72 percent of those delivered by cesarean section. Some extragenital causes of puerperal fever include respiratory complications, pyelonephritis, intense breast engorgement, bacterial mastitis, thrombophlebitis, and in cases of laparotomy, incisional wound abscess.
Respiratory complications most often are seen within the first 24 hours following delivery, and almost invariably are in women delivered by cesarean section or given general anesthesia for vaginal delivery. Complications include atelectasis which is best prevented with the use of routine coughing and deep breathing on a fixed schedule, usually every 4 hours for at least 24 hours following the administration of general anesthesia. Airway obstruction from thick secretions and diminished cough reflex increase this possibility. It is not conclusively proven that atelectasis causes fever (Engoren, 1995). Pyrexia is thought to be due to infection with normal flora that proliferate distal to the obstructing lesion. Roberts and colleagues (1988) reported that fever and x-ray findings of atelectasis correlate in only half of cases. Engoren (1995) found no association of fever and radiographic findings of atelectasis. Because of severe sequelae, the possibility of aspiration must be suspected in the woman with severe or persistent pulmonary findings.
Pyelonephritis may be difficult to diagnose postpartum. In the typical case, bacteriuria, pyuria, costovertebral angle tenderness, and spiking temperature clearly indicate renal infection; however, the clinical picture varies. For example, in the puerperal woman, the first sign of renal infection may be a temperature elevation, but costovertebral angle tenderness may not develop until later. The clinical diagnosis is confirmed by demonstrating bacteriuria microscopically and by urine culture. Empirical therapy is begun without waiting for culture results.
Breast engorgement commonly causes a brief temperature elevation. About 15 percent of all postpartum women develop fever from breast engorgement, rarely exceeding 39°C, in the first few postpartum days. The fever characteristically lasts no longer than 24 hours. In contrast, the elevated temperature of bacterial mastitis develops later and usually is sustained. It is associated with other signs and symptoms of breast infection that become overt within 24 hours.
Superficial or deep venous thrombophlebitis of the legs may cause mild temperature elevations in the puerperal woman. The diagnosis is made by the observation of a painful, swollen leg, usually accompanied by calf tenderness, or, occasionally, femoral triangle area tenderness. Treatment is given with intravenous heparin therapy.
Aspiration of acidic gastric contents during anesthesia for delivery can cause severe chemical pneumonitis. Aspiration is not limited to anesthesia for delivery. For example, treatment of eclampsia with large doses of morphine, barbiturates, or diazepam has been followed by the aspiration of gastric contents and a poor maternal outcome (Eclampsia Trial Collaborative Group, 1995). Management of anesthesia is discussed in Chapter 38.
INFECTIONS FOLLOWING CESAREAN DELIVERY
Delivery by cesarean section places the woman at extraordinary risk for developing uterine infection. Duration of labor and membrane rupture, multiple cervical examinations, and internal fetal monitoring are important determinants of infection morbidity. The incidence of metritis following surgical delivery varies with socioeconomic factors, and over the years this has been altered substantially by the common use of perioperative antimicrobial prophylaxis. Serious infections are much less common with the use of perioperative antimicrobials, whose use is discussed in detail in Chapter 35.
BACTERIOLOGY
Organisms that contaminate and invade surgical incisions and lacerations typically are those that normally colonize the cervix, vagina, and perineum. Most of these bacteria are of relatively low virulence, and seldom do they initiate infection in healthy tissues. Although more virulent bacteria may be introduced from exogenous sources, in modern obstetrics an epidemic of serious puerperal sepsis rarely develops. Serious infections with group A β-hemolytic streptococcus have been documented since the 1980s. This highly virulent organism may cause postpartum genital tract infections and a toxic shock-like syndrome (Nathan and coworkers, 1993; Whitted and colleagues, 1990). Udagawa and associates (1999) reviewed 30 cases of Group A infections in women either peripartum or postpartum. Of 17 in whom infection manifest before, during, or within 12 hours of delivery, maternal mortality was 88 percent from obvious infection and fetal mortality was 60 percent. In 13 women who deteriorated more than 12 hours postpartum, the maternal death rate was 55 percent and there were no fetal deaths.
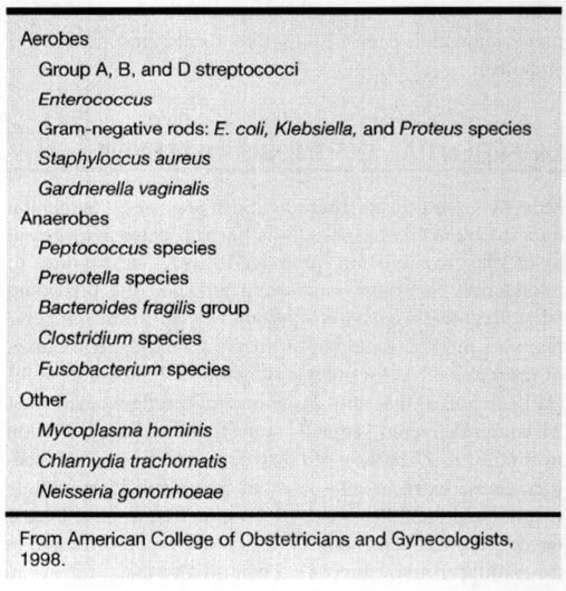
COMMON PATHOGENS. In the great majority of instances, bacteria responsible for pelvic infection are those that normally reside in the bowel and commonly colonize the perineum, vagina, and cervix (Gilstrap and Cunningham, 1979). Bacterial isolation and identification is a research endeavor and routine pretreatment genital tract cultures in women with puerperal infection are of little clinical utility (American College of Obstetrics and Gynecology, 1998). Bacteria commonly responsible for genital tract infections are shown in Table 17-1. Usually, multiple species of bacteria are isolated, and although typically considered to be of relatively low virulence, these bacteria may become pathogenic as a result of hematomas and devitalized tissue. Under the circumstances, their pathogenicity now is enhanced sufficiently to cause uterine infection with extensive pelvic cellulitis, abscesses, peritonitis, and suppurative thrombophlebitis.
TABLE 17-1. Bacteria Commonly Responsible for Female Genital Infections
The uterine cavity usually is sterile before rupture of the amnionic sac. As the consequence of labor and associated manipulations, amnionic fluid and presumably the uterus commonly become contaminated with anaerobic and aerobic bacteria. For example, Gilstrap and Cunningham (1979), from cultures of amnionic fluid obtained at cesarean section performed in women in labor with membranes ruptured more than 6 hours, identified an average of 2.5 organisms from each woman. The following bacteria were isolated: anaerobic and aerobic organisms in 63 percent, anaerobes alone in 30 percent, and aerobes alone in 7 percent. Predominant anaerobic organisms were gram-positive cocci (Peptostreptococcus and Peptococcus), 45 percent; Bacteroides, 9 percent; and Clostridium, 3 percent. Gram-positive aerobic cocci also were common (Enterococcus, 14 percent; group B Streptococcus, 8 percent). Escherichia coli comprised 9 percent of isolates.
These observations serve to emphasize the polymicrobial nature of genital infections associated with delivery, and especially cesarean section. Gibbs (1987) reemphasized the importance of these organisms, and reported an increasing prevalence of Bacteroides bivius as a cause of female pelvic infection. Walmer and colleagues (1988) also provided evidence for Enterococcus in the pathogenesis of these infections.
PATHOGENESIS
The pathogenesis of uterine infection following cesarean section is that of an infected surgical incision. Bacteria that colonized the cervix and vagina gain access to amnionic fluid during labor, and postpartum they invade devitalized uterine tissue. Invariably, with uterine infections that follow cesarean section, there is myometritis with parametrial cellulitis. Hence the term metritis is more descriptive than endometritis.
CLINICAL COURSE
The clinical picture of metritis varies with the extent of the disease, and whenever fever persists postpartum, uterine infection should be suspected. The degree of temperature elevation probably is proportional to the extent of infection, and when confined to the endometrium (decidua) and superficial myometrium, the cases are mild and there is minimal fever. With more severe infection, the temperature often exceeds 38.3°C. Chills may accompany fever and suggest bacteremia, which may be documented in nearly 20 percent of women with uterine infection following cesarean delivery. The pulse rate typically follows the temperature curve.
The woman usually complains of abdominal pain, and afterpains may be bothersome. There is tenderness on one or both sides of the abdomen and parametrial tenderness is elicited upon bimanual examination. Even in the early stages an offensive odor may develop, long regarded as an important sign of uterine infection. In many women, however, foul-smelling lochia is found without other evidence for infection. Conversely, some infections, and notably those due to group A β-hemolytic streptococci, frequently are associated with scanty, odorless lochia. Leukocytosis may range from 15,000 to 30,000 cells per μL, but in view of the physiologic leukocytosis of the early puerperium, these findings are difficult to interpret.
TREATMENT OF METRITIS
Postcesarean section metritis is treated with a parenterally administered broad-spectrum antimicrobial(s). Improvement will follow in 48–72 hours in nearly 90 percent of women treated with one of the regimens discussed below. The usual contemporary practice is to discharge the woman without further therapy after she has been afebrile for at least 24 hours (Dinsmoor and colleagues, 1991).
Persistence of fever after 72 hours mandates a careful search for causes of refractory pelvic infection, although nonpelvic sources occasionally are found. Complications of metritis that cause persistent fever despite appropriate therapy include parametrial phlegmons, surgical incisional and pelvic abscesses, and septic pelvic thrombophlebitis.
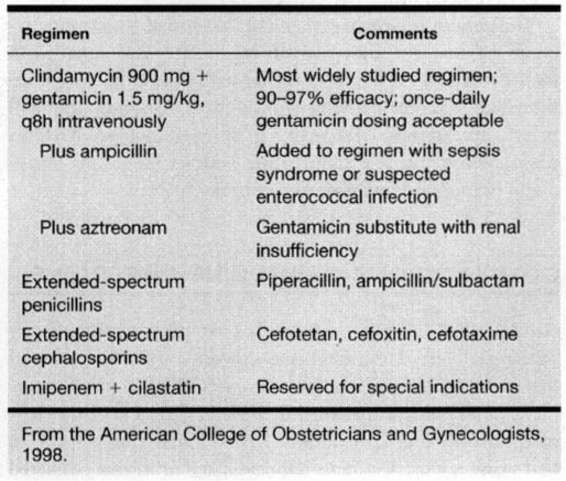
Initial treatment is directed against at least most of the polymicrobial and mixed flora listed in Table 17-1. Selection of an agent(s) effective against the most common pathogens usually proves suitable. Because, as described earlier, material for culture is impractical to obtain, antimicrobial therapy is empirical. A number of regimens with proven efficacy are listed in Table 17-2.
TABLE 17-2. Antimicrobial Regimens for Pelvic Infection Following Cesarean Delivery
In 1979, diZerega and colleagues compared the effectiveness of clindamycin plus gentamicin with penicillin G plus gentamicin given for treatment of pelvic infections following cesarean section. Women given the clindamycingentamicin regimen had a favorable response 95 percent of the time, and this regimen now is considered by most obstetricians to be the standard against which others are measured. Walker and colleagues (1988) later provided evidence that enterococcal infections may be associated with its clinical failure, thus, many add ampicillin to the clindamycingentamicin regimen, either initially or if there is no response by 48–72 hours.
Recently, the University of Alabama group (Brumfield and colleagues, 2000) reaffirmed the efficacy of this regimen given to 322 women with postcesarean metritis and pelvic cellulitis. Over half (54 percent) were cured with the original two-drug regimen, and another 40 percent in whom ampicillin was added at 48 hours responded. Of the 19 women (6 percent) who did not respond to “triple therapy,” seven had a wound infection that required drainage.
Regarding gentamicin, it is not necessary to measure peak and trough serum gentamicin concentrations in most women and once-daily dosing is now used by many. Because the potential for nephrotoxicity and ototoxicity is worrisome with gentamicin in the event of diminished glomerular filtration, such women can be given a combination of clindamycin and a second-generation cephalosporin. Also recommended is a combination of clindamycin and aztreonam, a monobactam compound with activity against gram-negative aerobic pathogens similar to the aminoglycosides (American College of Obstetricians and Gynecologists, 1998).
β-Lactam antimicrobials have spectra that include activity against many anaerobic pathogens and have been used successfully for decades to treat these infections. Many of the popular and effective multiagent regimens include a drug from this group. Some of these have been proven effective when used alone. Examples include some cephalosporins (cefoxitin, cefotetan, cefotaxime, and others) and extended-spectrum penicillins (piperacillin, ticarcillin, and mezlocillin). β-Lactam antimicrobials are inherently safe, and except for allergic reactions, they are free of major toxicity. Another advantage is the cost-effectiveness of administering only one drug. The β-lactamase inhibitors, clavulanic acid and sulbactam, have been combined with ampicillin, amoxicillin, and ticarcillin to extend their spectra, and these also have been proven effective.
Imipenem is a carbapenem that has broad-spectrum coverage against the majority of organisms associated with metritis. It is used in combination with cilastatin, which inhibits the renal metabolism of imipenem. Although this will be effective in the vast majority of cases of metritis, it seems reasonable to reserve it for more serious infections, especially in immunocompromised patients.
COMPLICATIONS OF UTERINE INFECTIONS
As described in their study of 322 women undergoing cesarean delivery, Brumfield and coworkers (2000) reported that metritis responds within 72 hours to appropriate treatment in 94 percent of cases. In a study from Parkland Hospital, Brown and colleagues (1999) found that only 1 in 650 women with postpartum metritis had fever that persisted for more than 5 days despite adequate antimicrobial therapy. A number of infection-related complications are responsible for persistent fever.
WOUND INFECTIONS
The incidence of abdominal incisional infections following cesarean delivery has been reported to range from 3 to 15 percent with an average of 7 percent (Faro, 1990). When prophylactic antibiotics are given, the incidence is 2 percent or less. According to Soper and coworkers (1992), wound infection is the most common cause of antimicrobial failure in women given therapy for metritis. Risk factors for wound infections include obesity, diabetes, corticosteroid therapy, immunosuppression, anemia, and hematoma formation.
Incisional abscesses that develop in women delivered by cesarean section usually cause fever beginning on the fourth postoperative day or so. In most cases, these are preceded by uterine infection, and there is persistent fever despite adequate antimicrobial therapy. Erythema and drainage also may be present. Organisms causing these infections usually are the same as those isolated from amnionic fluid at the time of cesarean section, but hospital-acquired pathogens must be suspected (Emmons and colleagues, 1988; Gilstrap and Cunningham, 1979).
TREATMENT. When cellulitis first manifests, treatment with antimicrobials may be effective. Because most women with incisional wound infections were given prophylactic antimicrobials intraoperatively or were treated for metritis, these incisional infections may be relatively mild. An obvious hematoma or abscess, however, requires drainage. In women in whom an abscess is not initially apparent, antimicrobial therapy and observation is appropriate. With continuing infection the incision will become tender and more swollen, and the woman will have persistent fever. In some cases, the wound opens spontaneously.
Surgical drainage includes all purulent material, including pus loculations. In most cases, the entire length of the wound must be explored to the fascia. Careful inspection for fascial integrity is paramount when the wound is first drained. If the fascial layer is intact, then debridement and local wound care is appropriate. In some cases, inspection and debridement are initially carried out in the operating room because extensive debridement and exploration requires major anesthesia.
After vigorous debridement of all necrotic tissue and drainage of purulent material, the wound cavity is packed with moistened sterile gauze. Some prefer to soak the gauze in povidone-iodine solution. This dressing should be changed at least daily, and preferably twice daily, with appropriate debridement at each dressing change.
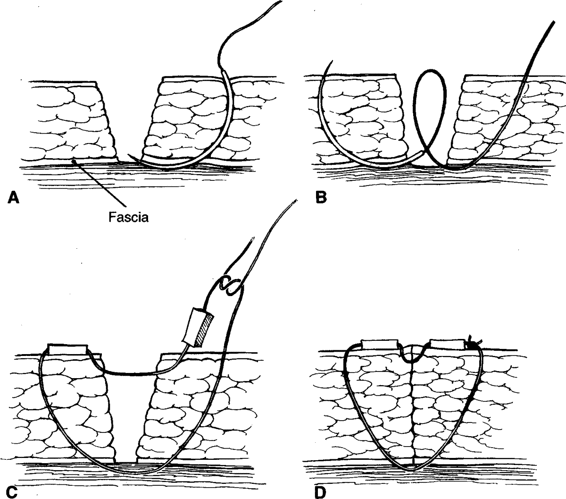
Healing by secondary intention takes several weeks to months depending on the size and depth of the wound. An aesthetically preferable method of management is to allow healthy pink granulation tissue to form—usually about 4–6 days—after which the wound edges are apposed with Steri-Strips or nonabsorbable suture. Walters and associates (1990) reported that the mean time to healing in six women randomized to second intention was 72 days. They recommended en bloc reclosure of disrupted incisions. The method, shown in Figure 17-1, was not employed for a minimum of 4 days after daily debridement and dressing changes three times a day. The size of the wound cavity is inconsequential, and if it is large because of excessive debridement of subcutaneous tissue, the wound edges can be approximated with Steri-Strips and the woman instructed to return in 1 week. If the strips come off, then she and her family are instructed to continue cleaning the wound as they were taught in the hospital. When she returns, Steri-Strips are replaced or suture material is used to approximate the skin edges.
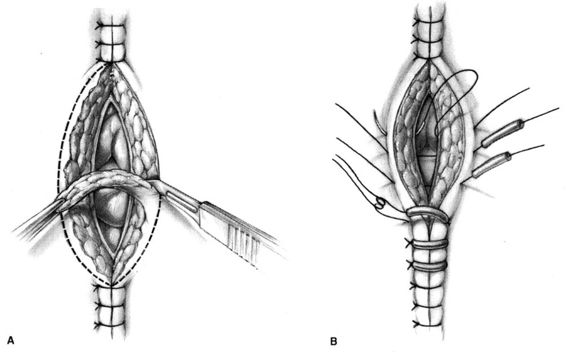
FIGURE 17-1. Technique of en bloc wound reclosure. A. The needle is passed from a point on the skin 3–4 cm from the wound edge through the superior part of the fascia at the wound base. B. The needle is withdrawn from the wound base, reintroduced into the fascia at the point from which it emerged with the first pass, and brought out through the skin at a point 3–4 cm from the opposite wound edge. C. Rubber suture guards are loaded onto the suture, the suture is brought across the incision incorporating the dermis and epidermis of the wound margins, and a second suture guard is loaded. D. The suture is tied, closing the wound. (Modified from Walters and colleagues, 1990, with permission.)
WOUND DEHISCENCE
Wound disruption, dehiscence, or “burst abdomen” refers to separation of the wound involving the fascial layer. McNeely and colleagues (1998) studied 8590 women undergoing cesarean delivery at Hutzel Hospital in Detroit. They reported that in 27 of these fascial dehiscence developed—about 1 in 300 operations. These closures are discussed in detail in Chapter 4.
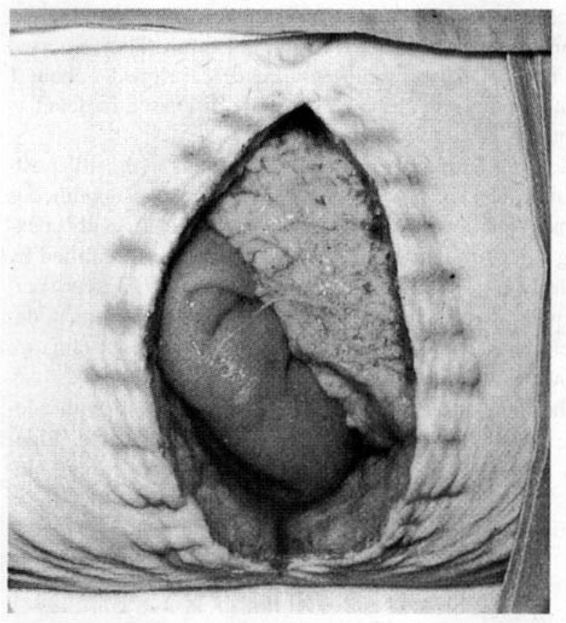
Most disruptions do not manifest until about the fifth postoperative day. The usual presenting sign is a serosanguineous discharge. Following cesarean delivery, there is usually coexisting infection. Of the 27 fascial dehiscences identified by McNeely and associates (1998), 18 (67 percent) were associated with infection and tissue necrosis. In some women, dehiscence becomes apparent when the skin sutures or clips are removed and evisceration follows (Fig. 17-2).
FIGURE 17-2. Wound dehiscence 7 days after cesarean section. After the skin sutures were removed, the wound fell apart. Bowel and omentum are apparent. (From Beischer and MacKay, 1981, with permission.)
The incision is closed in the operating room with adequate anesthesia. As Figure 17-3 shows, surgical debridement is carried out first, followed by secondary fascial closure. Most surgeons prefer myofascial approximation as described in Chapter 4 and shown in Figure 17-3.
FIGURE 17-3. A. Sharp debridement of abdominal wound closure of wound.
NECROTIZING FASCIITIS
This is an uncommon but serious complication of cesarean wound infections that is deep soft tissue infection involving muscle and fascia. Such infections may extend from any infection adjacent to myofascial edges; this includes surgical incisions or other wounds. They are seen also with vulvar infections in diabetic and immunosuppressed women, but they develop rarely in otherwise healthy women. In obstetrics, these severe infections are more commonly encountered in episiotomy and other perineal wounds (see Chap. 5).
At the University of Alabama, Goepfert and colleagues (1997) reviewed their experience with necrotizing fasciitis of the abdominal wall following cesarean delivery. From 1987 through 1994, there were 5048 cesarean deliveries and nine cases of fasciitis—1.8 per 1000 cesarean sections. In two women, including one with metastatic breast cancer, the infection was fatal.
Early skin changes usually include erythema, tenderness, and edema. Frequently, these are accompanying systemic symptoms of fever, malaise, and generalized toxicity. Initially the skin may be intact, but as gangrene progresses, the skin becomes discolored and vesicles and crepitus appear accompanied by cutaneous anesthesia and skin necrosis. In the Alabama series, the mean time from cesarean delivery to discovery of fasciitis was 10 ± 4 days (range 5–17 days).
Bacteria involved appear to be similar to those that cause other pelvic infections, but anaerobes predominate. Grampositive anaerobic cocci or Clostridium perfringens usually are isolated along with aerobic cocci, B. fragilis group or E. coli (Thompson and associates, 1993). S. pyogenes infections reappeared in the past decade as a cause for severe necrotizing infections (McHenry and colleagues, 1996; Nathan and Leveno, 1994; Rowan and North, 1995). Group B streptococci synergistically may cause necrosis (Schorge and associates, 1998; Sutton and colleagues, 1985).
Necrotizing fasciitis of the surgical incision may involve any of the several superficial or deep perineal fascial layers, and thus it may extend over the abdominal wall. Thompson and colleagues (1993) reported that these severe infections also might follow episiotomy, minilaparotomy, diagnostic laparoscopy, and suprapubic catheter placement. Clinical symptoms vary, and frequently it is difficult to differentiate superficial infections from deep fascial ones. A high index of suspicion, with surgical exploration if the diagnosis is uncertain, may be lifesaving. Stamenkovic and Lew (1984) recommend biopsy of the fascial edges with frozen section microscopic examination when the diagnosis is uncertain. Histopathologic findings from 17 women with clinical necrotizing fasciitis showed fasciitis in 15 and lobular and septal panniculitis in all 17. Certainly, if myofascitis progresses, the woman becomes very ill from septicemia, circulatory failure, and death.
TREATMENT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree