Fig. 36.1
Lateral radiograph of dynamic obstruction during inspiration for a child with subglottic (extrathoracic) airway obstruction from malacia. Notice how the airway caliber gets smaller during inspiration and returns to normal during exhalation
Further, most lesions in the extrathoracic space will manifest with similar clinical symptoms. As such, from simple clinical examination it is difficult to distinguish laryngeal edema from subglottic edema or stenosis, vocal cord dysfunction or paralysis, or laryngo- or tracheomalacia.
36.5 Quantifying the Degree and Location of Airway Obstruction
Unlike other respiratory diseases, children with significant upper airway obstruction often do not demonstrate respiratory failure on blood gas analysis (Wesley et al. 1968; Newth et al. 1972), and a normal blood gas is not necessarily reassuring if a patient has significant work of breathing. Therefore, the decision to implement further therapy or supportive measures such as intubation and mechanical ventilation is often based on clinical assessment. The addition of both invasive and noninvasive monitoring can help quantify the degree of work, the need for further therapy, and the response to a particular intervention (Argent et al. 2008a, b).
36.5.1 Pulsus Paradoxus
To maintain adequate flow, as resistance in the upper airway increases, the pressure differential between atmospheric pressure and intrathoracic pressure will necessarily have to increase. As a consequence, children with airway obstruction, at any level, will attempt to make their intrathoracic pressure more negative to ensure adequate airflow. This further decrease in intrathoracic pressure will result in increased afterload on the left ventricle by increasing left ventricular wall tension, without a significant change in LV preload. When the change in LV afterload is severe, cardiac output decreases, and the magnitude of arterial blood volume that is ejected during inspiration will decrease (Clark et al. 2004; Frey and Freezer 2001). While physiologic variation in cardiac output with the respiratory cycle is well described, when this response is exaggerated, it is known as pulsus paradoxus and can be visible on arterial or central venous pressure waveforms, as well as noninvasive pulse oximetry (Fig. 36.2). Moreover, the degree of pulsus paradoxus has been shown to correlate with the degree of airway obstruction (Clark et al. 2004; Steele et al. 1998). However, pulsus paradoxus is not unique to upper airway obstruction and may be present as a consequence of other respiratory or cardiac disease (Wong 2007; Swami and Spodick 2003; Yalamanchili et al. 2005; Blaustein et al. 1986; Shiomi et al. 1991; Tamburro et al. 2002).
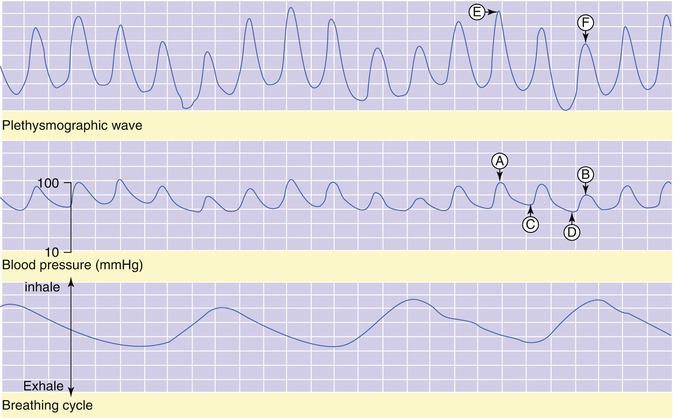

Fig. 36.2
Pulsus paradoxus in a child with croup. Points A and B represent systolic blood pressure, while points C and D represent diastolic blood pressure. Points E and F represent the peak amplitude of the pulse-oximetry waveform. Note that at the peak of inspiration (bottom), there is a decrease in both systolic and diastolic pressure (middle, D and B) and magnitude of the pulse-oximetry waveform (top, F)
36.5.2 Spirometry: Flow Versus Volume Loops
It is often difficult to determine whether a patient has upper airway obstruction upon extubation and can be even more difficult to determine the location of the airway obstruction—extrathoracic, intrathoracic, large airways, and medium- or small-sized airways. When combined with the physical exam, spirometry can help clinicians determine the level of obstruction and quantify its severity. This can be accomplished by placing either a tight-fitting mask over the nose and mouth for young children (Bates et al. 2000; Weiner et al. 2003) during tidal breathing or an oral tube with a good seal around the lips for older, cooperative children.
Patients with an extrathoracic obstruction, like croup, vocal cord dysfunction, or subglottic reactive edema after endotracheal intubation will manifest with flow limitation on inspiration, which has a characteristic flattening of the inspiratory limb of the flow-volume loop with a normal expiratory limb (Fig. 36.3). If the lesion is intrathoracic, then the shape of the flow-volume loop depends on whether the obstruction is fixed (such as foreign body aspiration, tracheal stenosis, vascular ring) or variable (tracheomalacia). Variable lesions like tracheomalacia generally show flattening on exhalation, while fixed lesions have flattening of the loop on both inspiration and exhalation. Diseases of the lower airways (asthma and bronchiolitis) classically have flow limitation on exhalation, with a characteristic “scooped” appearance to the expiratory limb of the flow-volume loop (Khemani et al. 2007).
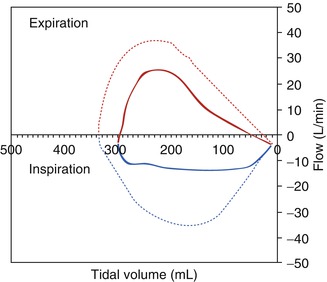

Fig. 36.3
Flow-volume loop for a child with extrathoracic airway obstruction. By convention, inspiration is negative on the Y axis and exhalation positive. Note the flattening of the inspiratory limb for the child with extrathoracic airway obstruction (solid) compared to the rounded normal (dotted) loop. Because the lesion is extrathoracic, the shape of the expiratory limb is not significantly different from a normal loop
36.5.3 Esophageal Manometry
Esophageal manometry has a variety of applications for titrating respiratory support and can be used as a surrogate marker for alveolar pressure and help compartmentalize compliance measurements for different components of the respiratory system (Benditt 2005). In addition, the relative change in esophageal pressure from inspiration to exhalation when combined with respiratory rate can be used as a surrogate marker for work of breathing (pressure-rate product (PRP)). While it does not directly measure work, PRP gives a relatively simple way to characterize the “effort” of breathing and correlates well with oxygen cost or metabolic work (Collett et al. 1985). Measurement requires placement of an esophageal catheter not much larger than a feeding tube into the lower third of the esophagus to measure intrathoracic swings in pressure (Fig. 36.4). The technique has been successfully applied to both infants and older children (Argent et al. 2008a, b; Graham et al. 2007; Willis et al. 2005; Klein and Reynolds 1986). While it does not distinguish the area of obstruction, it does quantify the respiratory effort and can be combined with spirometry to demonstrate inspiratory flow limitation with continual respiratory effort. A patient with extrathoracic airway obstruction (such as post-extubation stridor from reactive edema) will have a very characteristic pattern of flow and pressure (Fig. 36.5).
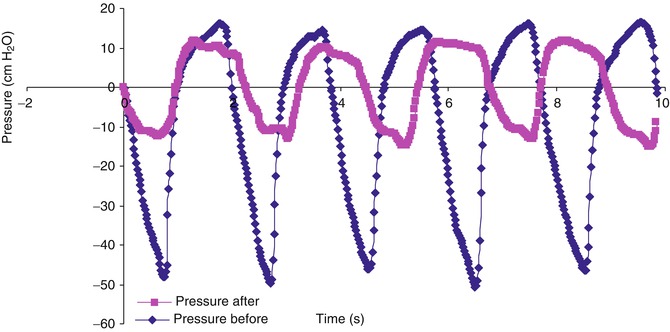
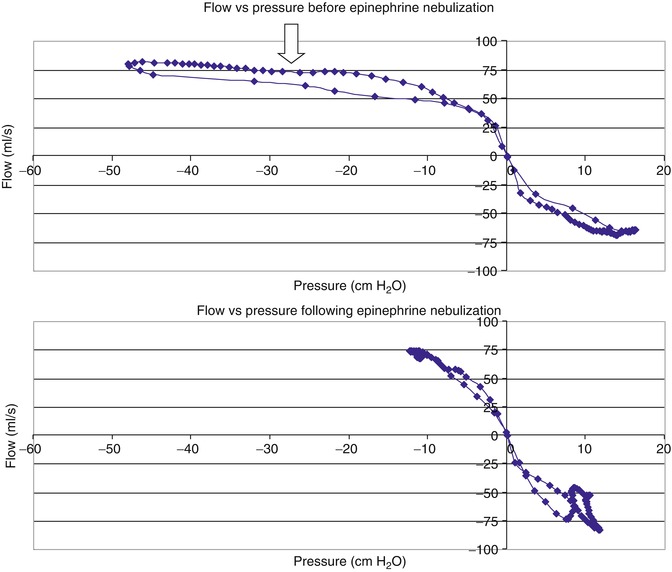

Fig. 36.4
Pressure versus time measured with esophageal manometry for a patient with extrathoracic airway obstruction from croup, before and after administration of racemic epinephrine. Note the decrease in change in esophageal pressure (ΔPes) and decrease in pressure-rate product (PRP = ΔPes * respiratory rate) from baseline (blue diamond) after racemic epinephrine administration (pink square)

Fig. 36.5
Flow-pressure curve for a child with extrathoracic airway obstruction from croup, before and after racemic epinephrine. The flow and pressure curves show inspiration on the left of the origin and exhalation on the right. Before epinephrine (top) there is flow limitation on inspiration as there is a continued decrease in esophageal pressure with no increase in flow (left of arrow). After epinephrine (bottom), flow increases and terminates without ongoing decreases in pressure
36.5.4 Respiratory Inductance Plethysmography
During normal tidal breathing, diaphragm contraction leads to inspiration, followed quickly by chest wall muscle activity. However, as respiratory pathology develops, there is often an increasing amount of thoracoabdominal asynchrony (TAA). This TAA can be characterized with respiratory inductance plethysmography in which elastic bands with an imbedded wire are placed around the rib cage and abdomen. A current is passed through the bands, which generates a magnetic field. The act of breathing alters the cross-sectional area of both the rib cage and abdominal components, thus altering the shape of the magnetic field generated by the bands. A computer can analyze this current and generate the phase angle, which is the degree of synchrony between the abdomen and rib cage, and the direction of the loop (Fig. 36.6) (Prisk et al. 2002). Studies on children with upper airway obstruction from croup (Sivan et al. 1990; Davis et al. 1993), as well as animal studies (Hammer et al. 1995), have demonstrated that the degree of TAA increases as the severity of UAO worsens. As such, phase angle measurements may serve as an objective measure to quantify the severity of airway obstruction and monitor response to interventions (Fig. 36.7). This technique offers the advantage of being completely noninvasive, unlike esophageal manometry. It is, however, not specific for UAO.
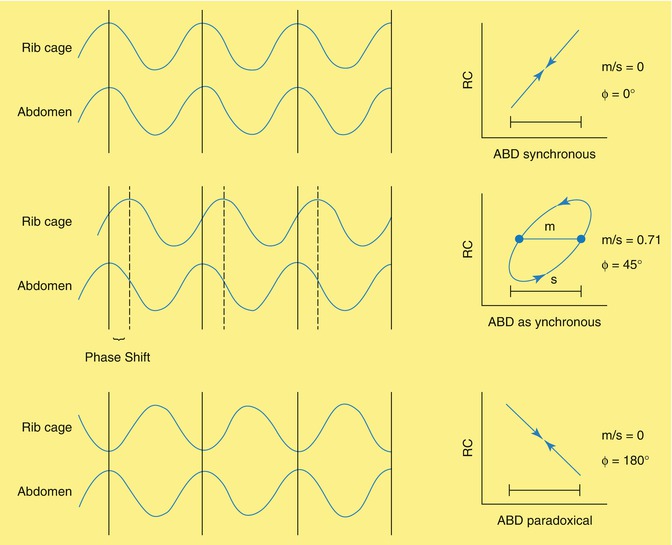
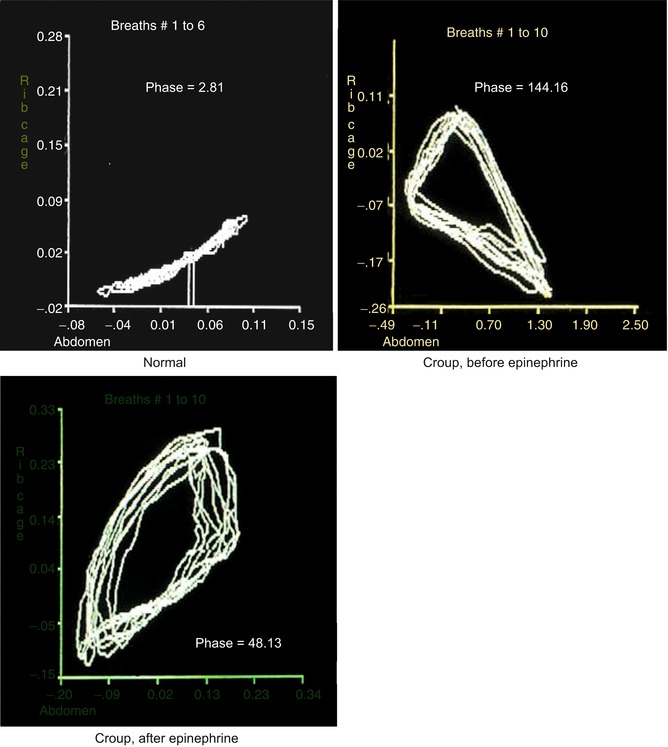

Fig. 36.6
Respiratory inductance plethysmography. Oscillatory waves are generated from the bands on the rib cage and abdomen. The phase angle is the arcsin [(width of the loop at the midpoint of the rib cage excursion)/(excursion of the abdomen)]. Under normal circumstances, the abdomen leads, followed very shortly by the rib cage, represented by a very small phase angle (Φ) with a very tight loop (top) and a counterclockwise rotation. With more asynchrony between the abdomen and rib cage, the phase angle will become larger, and the loop will take on a wider appearance (middle), although the direction of the loop remains counterclockwise. With complete paradoxical breathing (such as bilateral diaphragm paralysis), the rib cage and abdomen are exactly 180° out of phase, with clockwise movement of the loop with the rib cage leading

Fig. 36.7
Phase angles from respiratory inductance plethysmography for a child with upper airway obstruction from croup, before and after racemic epinephrine. Note that in the normal situation (top left), the abdomen and rib cage are synchronous, with a tight loop with a very small phase angle (phase = 2.81°). In the child with croup (top right), before administration of racemic epinephrine, there is more asynchrony, with a wide loop, and a large phase angle (144.16°). After racemic epinephrine (bottom left), the phase angle improves although the loop is not completely normal (48.13°) (Adapted from Sivan et al. (1990))
36.5.5 Endoscopy
While clinical estimates of post-extubation stridor may range from 6 to 30 %, endoscopic abnormalities may be present in up to 90 % of patients after extubation (Gomes Cordeiro et al. 2004). Single-institution studies have demonstrated that up to 50 % of infants and children may have minor changes such as edema, hyperemia, or mucosal erosion. Moderate lesions were seen in approximately 30 % of patients, including subglottic edema, vocal folds edema, ulceration, granulation tissue, or cartilaginous changes. Severe lesions including airway granulomas, subglottic stenosis, or vocal cord paresis were present in up to 10 % of patients. Of note, the reintubation rate secondary to upper airway obstruction was close to 25 % in this population, higher than most other centers. The long- and short-term clinical implications for such endoscopic findings are unclear, although other reports seem to indicate that children who suffer simply from airway edema seen on endoscopy continue to heal after extubation, while children with more severe airway abnormalities including tracheomalacia, vocal cord dysfunction, or subglottic stenosis may have more long-term morbidity (Lin et al. 2002).
36.6 Treatment and Prevention
36.6.1 Racemic Epinephrine
Racemic epinephrine has been used for many years for the treatment of acute infectious croup (Argent et al. 2008b; Westley et al. 1978; Kuusela and Vesikari 1988; Waisman et al. 1992). The mechanism of action is thought to be related to stimulation of alpha adrenergic receptors, causing local vasoconstriction in edematous mucous membranes, decreasing vasogenic edema. Because of its benefit in infectious croup, racemic epinephrine has been used to treat post-extubation stridor, although little formal evaluation of its use to prevent reintubation from upper airway obstruction has been done (Argent et al. 2008b; Davies and Davis 2002), with no randomized clinical trials. Nonetheless, in its nebulized form, it is commonly used in pediatric intensive care units for the treatment of post-extubation stridor. It can also be nebulized with the aid of continuous or intermittent noninvasive positive pressure ventilation or vaporized with high-flow humidified nasal cannula for continuous administration in the spontaneously breathing child (Obrien et al. 2007).
36.6.2 Heliox
Helium/oxygen mixtures can be delivered continuously via nasal cannula to improve laminar flow. Because the molecular weight of helium is lower than nitrogen, helium/oxygen mixtures can decrease the relative density of the gas mixture in a pipe (trachea), and lower the Reynolds number. When the Reynolds number is <2,300, flow is laminar, with lower airway resistance. Heliox has been shown to be effective in infectious croup (Weber et al. 2001) and can also help treat post-extubation stridor, although studies have typically been performed in specific pediatric subgroups such as burns (Rodeberg et al. 1995) or trauma (Kemper et al. 1991b).
36.6.3 Noninvasive PPV
Noninvasive positive pressure ventilation, through the form of nasal or mask continuous positive airway pressure (CPAP), bi-level support (BIPAP), or high-flow humidified nasal cannula, may prevent reintubation secondary to post-extubation stridor, when the upper airway obstruction is relatively mild (Ito et al. 2006; Sundaram and Nikolic 1996; Chantarojanasiri et al. 1993; Wang et al. 1996; Byerly et al. 2006). The mechanism of action is likely related to stenting of the upper airway with positive pressure and may facilitate delivery of medications, such as epinephrine. However, there has been no systematic assessment of the utility of such therapies, and their use has mostly been described in case reports.
36.6.4 Reintubation
When upper airway obstruction after extubation proves severe and does not respond to other noninvasive therapies such as racemic epinephrine, heliox, or noninvasive ventilation, reintubation may be necessary. It is generally believed that upon reintubation, a smaller endotracheal tube should be placed, again with care taken to ensure that an adequate air leak is present upon intubation with the head in midline position and the patient muscle relaxed. While many practitioners may place these patients on prophylactic corticosteroids before another extubation attempt, this practice has not been proven either effective or necessary (Harel et al. 1997).
36.6.5 Corticosteroids
On the grounds that the reactive edema which develops around an endotracheal tube may improve with the aid of anti-inflammatory agents, systemic corticosteroids have been used to try to treat or prevent post-extubation stridor for many years. While there has yet to be an adequately powered randomized controlled trial addressing the use of steroids to prevent post-extubation stridor in neonates or children (Tellez et al. 1991; Anene et al. 1996; Courtney et al. 1992; Couser et al. 1992; Ferrara et al. 1989), there is evidence that corticosteroids may be beneficial to prevent post-extubation stridor for “high-risk” children, particularly if given as multiple doses begun 12–24 h prior to extubation (Anene et al. 1996; Tibballs et al. 1992; Markovitz et al. 2008). However, there is not adequate evidence that reintubation from upper airway obstruction can necessarily be prevented with prophylactic corticosteroids. Unfortunately, the data for children are limited to only a few randomized controlled trials, which have significantly different results. The trials in neonates and children which have shown a benefit for steroids included children with underlying airway anomalies, multiple intubations, or airway trauma (Anene et al. 1996; Couser et al. 1992). While any corticosteroid could theoretically be used, dexamethasone given intravenously for 4–6 doses at 0.25–0.5 mg/kg/dose appears to be most accepted for infants and children (Markovitz et al. 2008).
There is growing evidence that for adults multiple doses of corticosteroids begun 12–24 h prior to extubation are beneficial to prevent post-extubation stridor and may be beneficial to prevent reintubation from airway obstruction, particularly if the patients are in some way “high risk” (Cheng et al. 2006; Francois et al. 2007; Lee et al. 2007; Markovitz et al. 2008; Khemani et al. 2009). Most “high-risk” criteria in adults involve patients who are intubated for a minimum of 24–48 h and fail certain cuff leak volume criteria (<24 %, or <110 ml).
Unfortunately, characterizing “high risk” in infants and children is a bit more challenging, particularly given that objective measures of upper airway obstruction are not routinely used in clinical practice. While several potential risk factors exist for the development of post-extubation stridor (see above), few if any of them have been evaluated prospectively to help define a high-risk cohort in which corticosteroids may be of more benefit. Further, given the clear differences in airway structure, anatomy, and intubation and ventilation practices between adults and children, extrapolating adult evidence regarding the use of corticosteroids to children or neonates is not warranted. However, future studies that address the issue of corticosteroids for the prevention of reintubation secondary to upper airway obstruction in neonates and children should employ objective criteria to identify high-risk groups and employ treatment regimens with multiple doses of corticosteroids begun 12–24 h prior to extubation.
36.7 Long-Term Complications of Endotracheal Intubation
With the growth of the use of endotracheal intubation and mechanical ventilation in the 1970s–1990s, the incidence of severe airway complications such as subglottic stenosis rose considerably, with rates ranging from 0.8 to 8 % (Wiel et al. 1997; Grundfast et al. 1990; Puhakka et al. 1990). With greater attention to appropriate tube sizing and sedation during intubation, there appear to be fewer reported cases of subglottic stenosis in the literature, although there has been surprisingly little published in the last several years. Nonetheless, even with great care and safe intubation practices, some children will still develop severe subglottic stenosis which may require further surgical consideration or tracheostomy. Other rare complications reported include neuromuscular dysfunction with tongue drop, laryngotracheal incoordination, saliva pooling over the larynx with poor cough reflex, laryngomalacia, tracheomalacia, airway granulomas, and vocal cord paralysis (Lin et al. 2002; Martins et al. 2006).
Essentials to Remember
Complications of post-extubation airway obstruction can be minimized by careful selection of an appropriate endotracheal tube. While population-based formulae should be used as a guide, endotracheal tube size should be personalized for the patient by assuring an appropriate air leak on initial placement.
Cuffed endotracheal tubes can be as safe as uncuffed endotracheal tubes, as long as care is taken to assure the tube is of appropriate size on initial placement. In addition, the cuff should only be inflated to the minimal amount necessary to guarantee adequate ventilation, still allowing for an air leak at or just above the set or generated peak inspiratory pressure.
The extubation air leak test has not been reliably associated with increased likelihood of extubation failure. While the presence of an air leak at or below 30 cm of H2O may be reassuring, the absence of a leak does not reliably mean the patient is more likely to fail extubation and should not delay extubation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


