Polysomnography and MSLT
Introduction
Cliched as it may be the wisdom in the art of medical evaluation of the sick lies primarily in a thorough history and clinical examination. The preceding chapters have aptly illustrated the complexities of evaluating the child with sleep disorders in this regard. Thus, the reader will have appreciated that several medical and non-medical conditions can lead to, or, even masquerade as, a sleep disorder. The reverse is certainly true as well. For instance, excessive daytime sleepiness (EDS) may present with depressive symptoms and obstructive sleep apnea syndrome (OSAS) as attention deficit disorder. As in all other branches of medicine, recourse to diagnostic testing is helpful to clarify clinical suspicions and confirm diagnoses. It is now clearly established by several studies that no combination of clinical history and physical examination will reliably distinguish the presence or absence of OSAS in a snoring child.1–3 This observation illustrates the need for confirmatory diagnostic testing, specifically polysomnography (PSG). Similarly, PSG is required for the diagnosis of rapid eye movement (REM) sleep behavior disorder where the absence of the skeletal muscle atonia normally present during REM sleep is useful to confirm clinical suspicion. Furthermore, the multiple sleep latency test (MSLT) is a standard tool to objectively ascertain the propensity of daytime sleepiness in patients with symptoms suggestive of EDS.
Alternate tools for the evaluation of the child with a sleep disorder do exist. For instance, with respect to the child with suspected OSAS (the commonest indication for PSG), it has been felt that due to a shortage of pediatric sleep testing centers in the United States or for financial reasons, substitute diagnostic tests such as home video monitoring, daytime nap testing, abbreviated sleep studies (such as overnight oximetry and portable home recordings) may be tried.4 However, the relatively lower predictive values of these tests make them less desirable when compared to the ‘gold standard’ PSG.3 Other tools often used in sleep clinics include a variety of sleep logs and questionnaires for sleep habit screening and to evaluate daytime sleepiness or circadian preferences.5 Actigraphs, which are wrist-watch-sized devices housing accelerometers, sense motion or lack thereof as a measure of rest and activity levels. These activity-reporting data are then useful as surrogate indicators of wake and sleep. Less commonly, HLA-markers as well as cerebrospinal fluid hypocretin levels are used in the evaluation of patients with EDS who are suspected to have narcolepsy.6,7
In this chapter, we will discuss the indications and techniques pertaining to PSG and MSLT, with special emphasis on infants and children. Technical details relative to the standards of clinical practice and laboratory space, laboratory protocols, instrumentation, and specific scoring rules are not discussed in detail in this chapter. The reader is directed to the official guidelines set forth in publications by the American Academy of Sleep Medicine (AASM).8–10 Excellent information on the requirements for maintaining accreditation of sleep diagnostic facilities is also available at the AASM’s website (http://www.aasmnet.org/accred_centerstandards.aspx). Furthermore, while the merits and standards of studying childhood sleep disorders in integrated adult and pediatric laboratories versus pediatric specific accredited laboratories is being hotly debated,11,12 this chapter will simply describe the traditional ‘attended in-lab’ types of sleep studies in a child-friendly setting.
Indications for Polysomnography
By far, the commonest reason for ordering a sleep study is to confirm the suspected diagnosis of OSAS and other types of sleep-related breathing disorders (SRBD) prior to any therapeutic intervention. In recent statements, the American Academy of Pediatrics and the AASM have outlined their positions on the diagnosis and management of OSAS and emphasized that follow-up PSG should be additionally performed after an intervention such as tonsilloadenoidectomy (T&A) in children with persisting symptoms as well as those at high risk for incomplete resolution of OSAS with T&A. Such children include those with severe preoperative OSAS, obesity, craniofacial anomalies impacting the upper airway, certain neurological conditions (e.g., meningomyelocele) and genetic disorders (e.g., Down syndrome). These children may require additional interventions for persisting SRBD such as positive airway pressure (PAP) therapy.3,13 Evidence is less robust for the use of PSG for suspected SRBD accompanying chronic lower airway diseases such as asthma, cystic fibrosis, pulmonary hypertension, bronchopulmonary dysplasia, or chest wall abnormality such as kyphoscoliosis.13 Non-respiratory indications for PSG include apparent life-threatening events (ALTE), periodic limb movement disorder (PLMD), REM sleep behavior disorder (RBD) and selected cases of childhood restless legs syndrome (RLS)14 (Table 49.1).
Table 49.1
Respiratory and Non-Respiratory Indications for and against PSG in Children
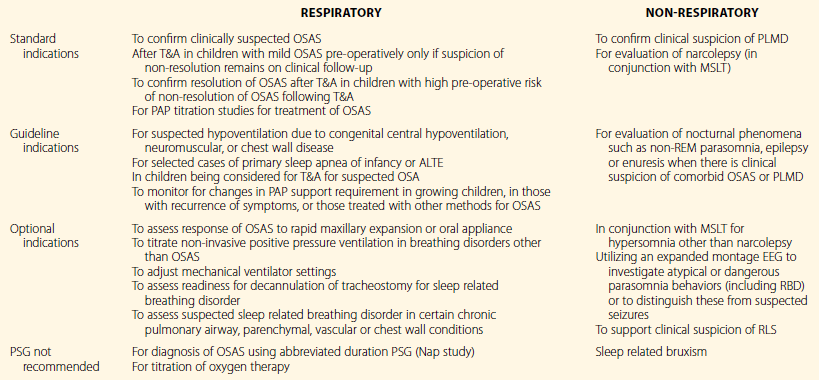
Adapted from Aurora RN, Zak RS, Karippot A, et al; American Academy of Sleep Medicine. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011; 34(3):379–88 and Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012; 35(11):1467–73.
Organization of Sleep Services in the Context of the Sleep Study
Equipment and Study Timing
While the pediatric sleep study is broadly very similar to its adult counterpart in the scope of the sleep disorders tested,15 several key differences are worthy of emphasis. Pediatric populations are diverse in age, and any sleep laboratory catering to childhood sleep disorders needs to be equally adept in monitoring a newborn infant as well as an older teenager. It is therefore essential to maintain the equipment to cater to these age groups in the inventory. This applies to the sensors (e.g., nasal cannula) as well as therapy (e.g., interfaces to deliver positive airway pressure).
The study may need to be timed to match the variable sleep times of children at different levels of maturity. Newborns will sleep for substantial periods in the day and planning of daytime PSGs may free up beds at night for the laboratory. Conversely, a delayed testing schedule may be needed to match the teenager apt to present with a circadian phase delay.16
Friendliness Factor
Perhaps the single most important factor that makes a sleep laboratory worthy of qualifying as a pediatric sleep laboratory is its ‘child friendliness factor.’ This applies not only to the physical space itself, but also extends most vitally to the friendliness of the technical and non-technical staff. Zaremba and colleagues have described the ingredients of such a setting very well.17 As soon as they enter the door, the child’s experience often begins to shape the likelihood of cooperation. The decor, the friendliness of the reception area and reassuring words, all go a long way in setting the stage. While teens should not be talked down to or patronized, the younger children may need repeated reassurance and gentle handling. Simple language should be used to describe the plan for the night. A ‘show and tell’ attitude for equipment needs to be borne in mind before the hook-up. The child should understand that the hook-up will be painless (‘no ouchies,’ ‘no needle sticks’) and the technologist must not neglect to assure the young patient that all equipment will come off painlessly next morning. A quick little demonstration with an EEG sticker application on a parent (with the child’s permission!) may be very reassuring to reinforce this fact as well as to enlist the child’s active participation in the process. Younger girls may be excited about wearing ‘shiny hair jewelry,’ whereas boys may like to hear they will look ‘like a space cadet’ once they are all hooked up with the EEG electrodes. Asking families to bring their cameras along to take ‘cool looking’ pictures adds to the excitement of ‘camping out at the sleep center!’; TV, DVD and other diversions help. The child may need reassurance that their parent will be in the room and that their favorite teddy bear can accompany them to bed (Figure 49-1).
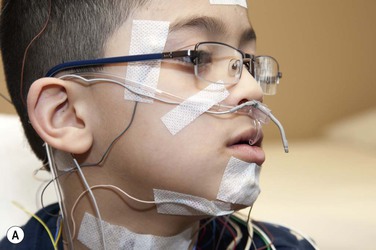
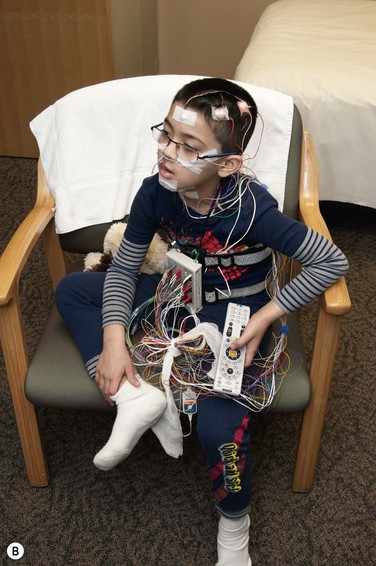
Figure 49-1 (A) This child has been hooked up for a polysomnogram. This close-up shows the placement of oro-nasal thermistor and transducer to capture airflow as well as measure ETCO2. Right ocular and chin electrodes covered with tape can be seen. The referential right mastoid lead can be seen behind the right pinna. A snore microphone is attached to the neck.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved.
(B) The non-threatening environment of this child friendly sleep center is apparent. This young child feels right at home and is able to watch his favorite DVD during and after his PSG hook-up. Visible scalp electrodes include ground (mid forehead), left frontal and central electrodes. Notice the stuffed toy animal by his side for comfort. The jack box hanging around his neck accepts all the electrodes allowing mobility. The jack box will be finally connected to the control room computers once the child is in bed. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved.
The beds used in the pediatric sleep laboratory have to be configurable to allow for a parent to room-in with the child in their own separate bed. Ability to bring in a crib or toddler bed for the younger patient or substitute these with a full-size bed for the teenager requires adequate planning for storage space for extra beds. Furthermore, beds have to have safety rails for the appropriate cases, and other safety cautions may have to be modified for the child.18 Finally, easy access to restroom facilities, odor-sealing diaper disposals, and accommodations for wheelchairs or special equipment for the medically challenged child all need to be considered while planning and designing the sleep center.
Pre-Planning the Logistics of the Study
All orders ideally should be carefully scrutinized by a knowledgeable person in the laboratory to ascertain that the study can be run as directed. For instance, usually one PSG technologist runs two studies at night in most adult settings. This is commonly referred to as 1 : 2 staffing. In the pediatric setting, however, due to the laborious nature of the task, especially in children with special needs or the younger child, a ratio of 1 : 1 is often required. Timing of the start of the PSG (‘lights out’) will be very different for a toddler versus a teenager who sleeps late, or a patient who is traveling multiple time zones to a referral center. Furthermore, sleep technologists often come with prior training in other fields. The supervising technologist will use this knowledge to ensure that the sleep technologist assigned to run any particular study is matched to the clinical need. Thus, a technologist with a respiratory therapy background may be more suited to run a ‘ventilator check’ while the child with complex parasomnia with suspected seizure disorder may be the forte of the EEG-trained technologist. For 1 : 2 staffing, the anticipated ‘easy hook-up’ may be paired with a ‘tough hook-up,’ or the early ‘lights out’ study be matched with a late sleeper to allow the technologist time to accomplish the studies on both patients more comfortably.
Preparing the Family for the Sleep Study
Any visit to the doctor is an anxiety-provoking experience for the child and the family. For most, the sleep study is a novel experience. Therefore, seeds for a successful study are best planted during the clinical visit. Careful explanation of the reason for the study and the types of sensors and their importance are crucial to enlist patient and parental cooperation during the PSG. For instance, while most children dislike the nasal cannula, a partnership with the parents helps provide reassurance to the child and transmits the expectation to keep the device on. Similar considerations apply to the other sensors as well. An informed parent is the technologist’s best ally when struggling with an uncooperative child at night. The use of sedation to accomplish the PSG is not recommended, and adverse events have been reported in children with OSA.19 Accordingly, the family may benefit from touring the laboratory a few days prior to the test night. This approach not only reduces anxiety, but also helps the parents and child to plan what clothing, personal effects, or entertainment items to bring along in their overnight bags to make the child comfortable at night. Besides, they get an opportunity to see the sleeping options for the accompanying parent.
The importance of maintaining a sleep schedule leading up to the sleep study should be discussed to avoid, for instance, that late-night party on Friday and consequently a non-representative study night on Saturday. Similarly, a late afternoon nap may delay sleep onset at night significantly. Parents should also be advised to reschedule the PSG if the child is sick. An acute upper airway infection may compromise the accuracy of OSAS diagnosis. Medications that may affect the sleep study should be discussed and an adequate wash-out period should be allocated prior to the sleep study. This is especially true if an MSLT study is to follow the next day, since several medications are known to compromise the quality of the MSLT by direct or withdrawal–rebound effect on the sleep architecture.9
Running the Polysomnogram
The PSG Montage
The standard overnight PSG measures sleep parameters utilizing limited electroencephalogram (EEG), electrooculogram (EOG), chin and leg electromyogram (EMG), electrocardiogram (EKG), respiratory effort at the chest and abdomen via respiratory inductance plethysmographic (RIP) belts, pulse oximetry, capnometry via end-tidal or transcutaneous measurement (ETCO2, TcpCO2), and oro-nasal airflow via pressure transducer and thermistor (see Figure 49-1A and B).
The EEG electrode placement is based on an imaginary grid on the head measured in a standard fashion using the widely accepted 10–20 system.20 While specialized sleep centers may occasionally utilize the more extensive EEG montage essential for seizure diagnosis, only frontal (F), central (C), occipital (O) and eye (E) exploring electrodes are used to capture EEG and EOG data during the standard PSG. These are referenced to the contralateral mastoid (M) electrode on either side. Standardized numbering of these electrodes is based on location (e.g., C3, C4) with odd numbers represented on the left side by convention (Figure 49-2). EOG leads help monitor rapid eye movements (REM) of dream sleep (Figure 49-3). When combined with EMG data from chin leads, these channels allow for the scoring of sleep and wake into several stages (Figure 49-4). Each stage has several key characteristics that are well described elsewhere.8 Briefly, stage Wake shows some eye movements, persistent EMG tone in the chin, and age-appropriate dominant posterior rhythm in the occipital leads when the eyes are closed. Stage N1 is characterized by slow rolling eye movements and vertex sharp waves in the central leads. This gives way to sleep spindles and K-complexes characteristic of stage N2 as sleep progresses. Stage N3 is the deepest stage of sleep and comprises abundant delta waves which are 0.5–2 Hz in frequency and 75 µV in amplitude. Stage REM is scored when rapid eye movements are seen alongside mixed-frequency low-voltage EEG and importantly, a low EMG tone. Saw-tooth waves are characteristic as well. Thus, Wake, N1, N2, N3, and REM sleep stages can be scored throughout the sleep record. The EEG and EMG are also used to score arousals which are defined as 3 second long EEG frequency shifts in any sleep stage. There needs to be an accompanying EMG increase in REM stage, however. Arousals in turn are also of importance in the scoring of hypopneas.21
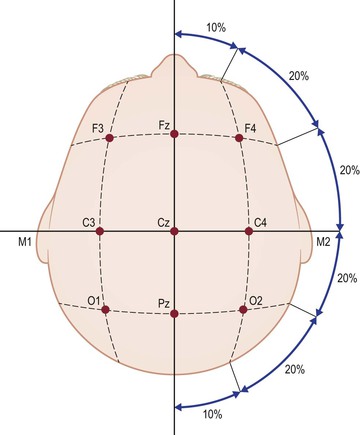
Figure 49-2 The International 10–20 system of electrode placement utilizes bony landmarks on the skull to delineate an imaginary grid which is numbered using standard nomenclature as shown. Measurements are made as a fraction of the total head circumference. See text for details. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


