Women with polycystic ovarian syndrome (PCOS) present with several endometrial abnormalities possibly explaining some of the adverse endometrium-related outcomes in these women. PCOS and an increased miscarriage rate have been suggested to coincide, but the results are conflicting. Recent studies have also shown increased risks of pregnancy-induced hypertension, preeclampsia, and premature delivery that may be related to altered decidualization/placentation in affected women. In the long run, PCOS per se is associated with the occurrence of endometrial cancer (EC), with obesity aggravating the risk.
Most investigated markers of the endometrial abnormalities in women with PCOS are related to steroid hormone action (ERs (estrogen receptors), PRs (progesterone receptors), ARs (androgen receptors), and steroid receptor coactivators), endometrial receptivity/decidualization ( HOXA10, αvβ3 integrin, and IGFBP-1 (insulin-like growth factor-binding protein 1)), glucose metabolism (IRs (insulin receptors), glucose transporters, IGFs) and inflammation/immune cell migration ((IL-6 (interleukin 6), CCL2 (C C motif ligand), and uNK (uterine natural killer) cells). Despite several endometrial abnormalities in women with PCOS, the clinical relevance of these findings still awaits future clarification; to date, no common screening protocols/recommendations for women with PCOS have been established.
Endometrial dysfunction in women with pcos
Endometrial pathologies related to reproductive functions
Several studies suggest an abnormal endometrial phenotype and function in women with polycystic ovary syndrome (PCOS) . In the past years, several investigators reported an increased risk of early miscarriage in women with PCOS as one of the first clinical findings ; however, not all studies agree . Some investigators report PCOS per se as an independent risk factor of miscarriage ; however, obesity and assisted reproductive technologies (ARTs), and recently hyperhomocysteinemia, have been shown to be associated with this risk in these women . While different PCOS phenotype-specific analyses are limited, oligo-amenorrhea has been suggested as one of the factors increasing the rate of miscarriage in affected women, whereas polycystic ovaries do not seem to add to the risk .
Adverse pregnancy outcomes have also been associated with PCOS. Affected women have been shown to present with a three- to fourfold increased risk of pregnancy-induced hypertension and preeclampsia and a twofold higher chance of premature delivery . Given this, it is likely that severe PCOS and its metabolic phenotype (amenorrhea, hyperandrogenism, obesity, diabetes, and hypertension) can affect the periconceptional endometrial milieu, leading to impaired placentation and promoting pregnancy complications that are also suggested to have an effect on the offspring ( Fig. 1 ). In fact, some adverse outcomes may be derived from abnormal trophoblast invasion and placentation in cases of PCOS, and surprisingly altered placental morphology has been reported even in spontaneous, uncomplicated PCOS pregnancies . Regarding the ART outcomes, a recent study showed an association between the increased number of oocytes (a common finding in women with PCOS undergoing gonadotropin stimulation) retrieved in cases of IVF (in vitro fertilization) and preterm birth and low birth weight; this again suggesting that abnormal placentation and an altered endometrial milieu may contribute to these events . Future elective embryo cryopreservation studies may partly elucidate the role of endometrial stress related to the IVF treatments and ovarian hyperstimulation and the role of endometrial health in cases of PCOS .

Endometrial cancer risk in women with PCOS
Women with PCOS present with a three- to fivefold increased risk of endometrial cancer (EC), the most common gynecological cancer in women . In the general population, EC affects about 25/100,000 women per year, with an increasing incidence corresponding to population aging, a Western lifestyle, and increasing obesity ( Fig. 2 A). EC is associated with endometrial hyperplasia, unopposed estrogen action, and genetic alterations and is classically divided into two subtypes: type I, most prevalent which has an estrogen-dependent nature, and a more aggressive kind, type II, which is commonly thought to be estrogen independent. Although type I shows a strong correlation with obesity, recent studies have shown that both cancer types share similar etiological factors, for example, low parity, low oral contraceptive use, cigarette smoking, early age at menarche, and diabetes .

Most importantly, previous studies have shown PCOS as an independent risk factor of EC (OR BMI-adjusted 2.4 (1.0–6.2)), whereas obesity, with a high prevalence among women with PCOS, further augments the risk (OR crude 4.3 (1.8–10.2)) ( Fig. 2 B). The etiology and pathogenesis of EC in women with PCOS are multifaceted, and the risk has been suggested to be related to oligo-amenorrhea, obesity, hyperandrogenism, hyperinsulinemia, abnormal luteinizing hormone (LH) secretion, inflammation, and genetic predisposition ( Fig. 3 ). Interestingly, the phenotype of EC has been shown to be somewhat different in women with PCOS compared with those without the condition. First, in the general female population, postmenopausal status is highly associated with EC, as the mean age at EC diagnosis is about 70 years, whereas the risk in women with PCOS is elevated among women aged <50 years . Given that the hormonal and metabolic dysfunctions are already present in PCOS at a young age, sometimes even from puberty, diagnosis of cancer at an early stage in affected women is expected. Secondly, PCOS is associated with type I (rather than type II) EC, which is commonly thought to be caused by long-term exposure to unopposed estrogen action and mostly encountered with obesity, diabetes, and hypertension, which are common comorbid conditions associated with PCOS . In line with this, some investigators have reported an increased rate of diagnosis of endometrial hyperplasia in women with PCOS . From a clinical viewpoint, adding obesity to the equation, it must be noted that the incidence of EC in women with PCOS is at least as high as the risk of breast cancer in the general population ( Fig. 2 B). Moreover, it should be noted that there is a risk of misdiagnosing the early manifestation of EC or hyperplasia in younger women with PCOS and diagnosing cancer-related menstrual irregularities as irregular bleeding episodes that are common in affected women. In future, further studies with rigorous histological examination and mechanistic approaches are warranted in order to reveal the possible PCOS-related EC characteristics and phenotypes to enable early diagnosis and prevention guidelines .
Normal endometrial morphology and function
Endometrial morphology and steroid hormone action in human endometrium
The endometrium lining consists of a simple columnar epithelium that is closely connected to the underlying stromal cells, where the epithelial glands are surrounded by stroma, thereby enabling complex interaction between these two cell compartments ( Fig. 3 A). Moreover, the stromal compartment beholds a great amount of resident and migrating immune cells as well as endothelial cells covering the small vessels. Recently, a new cell population with a perivascular location was discovered in the human endometrium, showing stem cell properties and possibly participating in the monthly renewal of the endometrial lining .
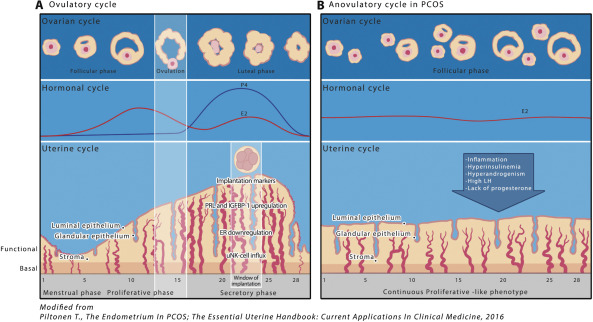
During normal menstrual cycles, the endometrium responds to the fluctuating levels of ovarian-derived steroid hormones (progestogens, androgens, and estrogens) dividing the endometrium monthly life cycle into proliferative and secretory phases (early, mid, and late) ( Fig. 3 A). Steroid hormones target the main cell types of the endometrium, that is, the epithelial and stromal cells, and they also have an impact on residing and transient immune cell activity and migration . The proliferative phase is characterized by an extensive estrogen-driven proliferation and mitotic activity, with the rising estrogen concentrations promoting the expression of estrogen receptors α (ERα) and β (ERβ), which are highest during the late proliferative phase . Estradiol (E 2 ), mainly through ERα activation, increases progesterone receptor (PR) expression, especially toward the late proliferative phase, thus enabling progesterone action on the endometrium after ovulation which in turn initiates the secretory phase of the endometrial cycle ( Fig. 3 A). Conversely, progesterone inhibits endometrial ER expression and thus estrogen action, especially in the epithelial glands, thus initiating endometrial reprogramming that induces differentiation (decidualization) in the stromal compartment.
Decidualization is mandatory for successful embryo implantation. It is dependent on the activation of cyclic adenosine monophosphate (cAMP) and progesterone signaling pathways and inhibition of estrogen signaling, with decreased cellular proliferation and DNA synthesis and reduction of cellular mitotic activity. Successful decidualization leads to morphological and biochemical transformation, including glandular secretion, large-scale remodeling of the vasculature, and influx of uterine natural killer (uNK) cells ( Fig. 3 A). As a result, the decidualized endometrium is able to adopt or abandon an embryo, regulate trophoblast invasion, and control inflammatory signals to sustain a healthy pregnancy . The homeobox (HOX) gene expression mediates steroid hormone (E 2 and progesterone) action and thus the decidualization process. In humans, the decidualized stromal cells classically present with high-level expression of HOXA10 and HOXA11 , prolactin (PRL), and insulin-like growth factor-binding protein 1 (IGFBP-1). When implantation does not occur, the corpus luteum collapses, leading to progesterone withdrawal from the differentiated endometrium and triggering a large-scale process with apoptosis and inflammatory cascades that culminate in shedding of the tissues and menses ( Fig. 3 A).
Besides ERs and PRs, human endometrium also expresses androgen receptors (ARs) in glandular and luminal epithelium and stroma. During the human menstrual cycle, ARs are upregulated by E 2 , similar to PRs, whereas progesterone and epidermal growth factor (EGF) downregulate ARs . Thus, AR expression is highest at the proliferative phase , thus decreasing toward the late secretory phase . Furthermore, 5α-reductase, the enzyme that converts testosterone (T) to the bioactive form, dihydrotestosterone (DHT; both being ligands for AR), is also expressed in normal endometrial cells . Interestingly, T concentrations peak around the time of implantation in the endometrium, suggesting the involvement of androgens in the endometrial differentiation process . Supporting for the role of AR and androgen action in endometrial physiology, the AR-knockout mice (ARKO mice) have endometrium-related subfertility and abnormal placental function well before ovarian failure . Indeed, ARs have been reported to mediate the decidualization of human endometrial stromal fibroblast (eSF) cytoskeletal organization and cell cycle regulation. In keeping with this, AR activation maintains decidualization and DHT augments this process in eSFs . Interestingly, a recent meta-analysis concluded that DHT therapy in poor responders in IVF resulted in increased live birth rates . Whether the beneficial effect is related to the direct improvement in endometrial function, and not only improved follicular development, remains to be determined .
Implantation
A well-balanced steroid hormone action on human endometrium prepares the mucosal lining for successful embryo attachment and trophoblast invasion. Approximately 6 days after ovulation, the endometrium is ready to receive the embryo and the window of implantation (WOI), endometrium displaying a receptive phenotype and a distinct gene profile, is opened for about 4 days ( Fig. 3 A). A number of molecular markers have been shown to be crucial for normal implantation. As mentioned earlier, among others, homeobox genes, especially HOXA10 and 11, are essential for endometrial growth, differentiation, and receptivity by mediating steroid hormone effects . In mice, HOX10- or 11 -knockout animals produce normal embryos that are vital in surrogate uterus; however, wild-type embryos do not implant in these knockout mice . Other endometrial receptivity-related mediators are also regulated by HOX genes, such as αvβ3 integrin, the previously mentioned IGFBP-1, and leukemia inhibitory factor (LIF) . During the normal embryo implantation process, the conceptus is able to enhance the endometrial response and increase the expression of decidual markers. Interestingly, a recent study revealed that the decidualized endometrial fibroblasts play an active role in embryo selection through an inflammatory stress response . Thus, in some women, an altered stress reaction toward normal or abnormal embryos could explain impaired endometrial function and non-optimal implantation.
Normal endometrial morphology and function
Endometrial morphology and steroid hormone action in human endometrium
The endometrium lining consists of a simple columnar epithelium that is closely connected to the underlying stromal cells, where the epithelial glands are surrounded by stroma, thereby enabling complex interaction between these two cell compartments ( Fig. 3 A). Moreover, the stromal compartment beholds a great amount of resident and migrating immune cells as well as endothelial cells covering the small vessels. Recently, a new cell population with a perivascular location was discovered in the human endometrium, showing stem cell properties and possibly participating in the monthly renewal of the endometrial lining .
During normal menstrual cycles, the endometrium responds to the fluctuating levels of ovarian-derived steroid hormones (progestogens, androgens, and estrogens) dividing the endometrium monthly life cycle into proliferative and secretory phases (early, mid, and late) ( Fig. 3 A). Steroid hormones target the main cell types of the endometrium, that is, the epithelial and stromal cells, and they also have an impact on residing and transient immune cell activity and migration . The proliferative phase is characterized by an extensive estrogen-driven proliferation and mitotic activity, with the rising estrogen concentrations promoting the expression of estrogen receptors α (ERα) and β (ERβ), which are highest during the late proliferative phase . Estradiol (E 2 ), mainly through ERα activation, increases progesterone receptor (PR) expression, especially toward the late proliferative phase, thus enabling progesterone action on the endometrium after ovulation which in turn initiates the secretory phase of the endometrial cycle ( Fig. 3 A). Conversely, progesterone inhibits endometrial ER expression and thus estrogen action, especially in the epithelial glands, thus initiating endometrial reprogramming that induces differentiation (decidualization) in the stromal compartment.
Decidualization is mandatory for successful embryo implantation. It is dependent on the activation of cyclic adenosine monophosphate (cAMP) and progesterone signaling pathways and inhibition of estrogen signaling, with decreased cellular proliferation and DNA synthesis and reduction of cellular mitotic activity. Successful decidualization leads to morphological and biochemical transformation, including glandular secretion, large-scale remodeling of the vasculature, and influx of uterine natural killer (uNK) cells ( Fig. 3 A). As a result, the decidualized endometrium is able to adopt or abandon an embryo, regulate trophoblast invasion, and control inflammatory signals to sustain a healthy pregnancy . The homeobox (HOX) gene expression mediates steroid hormone (E 2 and progesterone) action and thus the decidualization process. In humans, the decidualized stromal cells classically present with high-level expression of HOXA10 and HOXA11 , prolactin (PRL), and insulin-like growth factor-binding protein 1 (IGFBP-1). When implantation does not occur, the corpus luteum collapses, leading to progesterone withdrawal from the differentiated endometrium and triggering a large-scale process with apoptosis and inflammatory cascades that culminate in shedding of the tissues and menses ( Fig. 3 A).
Besides ERs and PRs, human endometrium also expresses androgen receptors (ARs) in glandular and luminal epithelium and stroma. During the human menstrual cycle, ARs are upregulated by E 2 , similar to PRs, whereas progesterone and epidermal growth factor (EGF) downregulate ARs . Thus, AR expression is highest at the proliferative phase , thus decreasing toward the late secretory phase . Furthermore, 5α-reductase, the enzyme that converts testosterone (T) to the bioactive form, dihydrotestosterone (DHT; both being ligands for AR), is also expressed in normal endometrial cells . Interestingly, T concentrations peak around the time of implantation in the endometrium, suggesting the involvement of androgens in the endometrial differentiation process . Supporting for the role of AR and androgen action in endometrial physiology, the AR-knockout mice (ARKO mice) have endometrium-related subfertility and abnormal placental function well before ovarian failure . Indeed, ARs have been reported to mediate the decidualization of human endometrial stromal fibroblast (eSF) cytoskeletal organization and cell cycle regulation. In keeping with this, AR activation maintains decidualization and DHT augments this process in eSFs . Interestingly, a recent meta-analysis concluded that DHT therapy in poor responders in IVF resulted in increased live birth rates . Whether the beneficial effect is related to the direct improvement in endometrial function, and not only improved follicular development, remains to be determined .
Implantation
A well-balanced steroid hormone action on human endometrium prepares the mucosal lining for successful embryo attachment and trophoblast invasion. Approximately 6 days after ovulation, the endometrium is ready to receive the embryo and the window of implantation (WOI), endometrium displaying a receptive phenotype and a distinct gene profile, is opened for about 4 days ( Fig. 3 A). A number of molecular markers have been shown to be crucial for normal implantation. As mentioned earlier, among others, homeobox genes, especially HOXA10 and 11, are essential for endometrial growth, differentiation, and receptivity by mediating steroid hormone effects . In mice, HOX10- or 11 -knockout animals produce normal embryos that are vital in surrogate uterus; however, wild-type embryos do not implant in these knockout mice . Other endometrial receptivity-related mediators are also regulated by HOX genes, such as αvβ3 integrin, the previously mentioned IGFBP-1, and leukemia inhibitory factor (LIF) . During the normal embryo implantation process, the conceptus is able to enhance the endometrial response and increase the expression of decidual markers. Interestingly, a recent study revealed that the decidualized endometrial fibroblasts play an active role in embryo selection through an inflammatory stress response . Thus, in some women, an altered stress reaction toward normal or abnormal embryos could explain impaired endometrial function and non-optimal implantation.
Markers of endometrial pathologies in women with PCOS
In recent years, an increasing number of studies have revealed several endometrial characteristics/markers related to the PCOS phenotype possibly explaining some of the unfavorable endometrium-related clinical manifestations. Owing to the heterogeneous nature of the PCOS population, some of the findings may only be manifested in the most severe cases and thus it is difficult to separate the effects of obesity, hyperinsulinemia, and inflammation, for example, from the syndrome itself. Nevertheless, the following sections will address findings/markers that have been shown to be differently expressed in the endometria of women with PCOS compared with the non-affected women. In some studies, the obesity issue has been addressed, whereas in others, it has not been considered necessary, as obesity is commonly present in cases of PCOS (40–80% of the women) and it is also strongly associated with the occurrence of the syndrome ( Table 1 ).
| Marker | PE | SE | HP | |
|---|---|---|---|---|
| Glucose metabolism | ||||
| IGFBP-1 | ▼ | SE (in vitro)▼ (Piltonen 2015) | ||
| GLUT 4 | ▼ | ▼ | PE▼, HP▼ (Li 2015); PE▼ (Fornes 2010); Zhai PE▼ (Zhai 2012*); PE▼ (Ujvari 2014*) | |
| IRS-1 | ▼ | PE▼ (Fornes 2010); PE▼ (Ujvari 2014*) | ||
| Glucose action | ▼ | PE▼ (Kim 2010*); PE▼ (Piltonen 2013*) | ||
| Inflammation | ||||
| IL-6 | △ | △ | PE△ (Piltonen 2013*); SE (in vitro)△ (Piltonen 2015) | |
| CCL2 (MCP-1) | △ | PE △(Piltonen 2013*) | ||
| IL-8 | △ | △ | PE△ (Piltonen 2013*); SE (in vitro)△ (Piltonen 2015) | |
| RANTES | △ | SE (in vitro)△ (Piltonen 2015) | ||
| uNK cells | ▼ | SE▼(Matteo 2010*) | ||
| MMPs | ||||
| MMP2 | △ | SE (in vitro)△ (Piltonen 2015) | ||
| MMP3 | △ | SE (in vitro)△ (Piltonen 2015) | ||
| Steroid hormone action | ||||
| HOXA10 | ▼ | SE▼(Taylor JCEM), Downregulated in EC (ref) | ||
| AR | △ | △ | △ | PE(-) (Piltonen 2013*); SE △ (Quezada 2006 FS*); PE△, HP△ (Li 2015); HP△(Villavicencio 2006) |
| PR | △ | SE △(Margarit 2010*) | ||
| ERα | △(-) | △ | △ | PE(-) (Piltonen 2013*); PE(-) (Kim 2010*); SE△(Gregory 2002) |
| PE?△ and HP△(Villavicencio 2006); SE△(Quezada 2006 FS*); SE△(Margarit 2010*) | ||||
| ERβ | △ | (-) | △ | SE (-) (Quezada 2006 FS*); PE?△ and HP△(Villavicencio 2006) |
| (avb3) integrin | ▼ | ▼ | PE▼ (Kim 2010*); SE▼(Quezada 2006 FS*); ▼progestin treatment (Lopez 2014) | |
| MUC1 | △▼ | SE△ovulatory PCOS,▼anovulatory PCOS (Margarit 2010*) | ||
| Steroid horomone co-activators | ||||
| AIBI | △ | △ | PE?△(Villavicencio 2006); SE△(Gregory 2002) | |
| TIF2 | △ | SE△(Gregory 2002) | ||
| NCoR | (-) | PE?(-) (Villavicencio 2006) | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


