Jose R. Valle, MD
Gulshan Sharma, MD, MPH
NORMAL MATERNAL PULMONARY PHYSIOLOGY
CLINICAL PRESENTATION AND INITIAL WORK-UP
OVERALL MANAGEMENT AND ADDITIONAL CONSIDERATIONS
ETIOLOGY AND SPECIFIC TREATMENT
1. Clinical Signs and Symptoms of Tuberculosis
2. Latent Tuberculosis Infection Screening
3. Active Tuberculosis Screening
INTRODUCTION
Respiratory infection during pregnancy and peripartum period is a complex condition with specialized concerns in terms of risks, diagnosis, and management. Although the pathogens are often similar to those seen in the general population, pregnant patients compose a unique group because of altered physiology and differences in immunologic response, leading to singular risk profile. The chances of associated morbidity and mortality, not only for the patient but also the fetus, are increased and require clinicians to adjust their management as needed. In fact, pneumonia is an independent risk factor for preterm labor and delivery, cesarean delivery, smaller size for gestational age, lower birth weights, lower Apgar scores, and preeclampsia/eclampsia.1–4
This chapter introduces normal maternal pulmonary physiology, diagnosis and management of respiratory infections, and special therapeutic considerations in the pregnant patient. Although obstetric patients may have significant respiratory and systemic symptoms related to upper respiratory tract infections, such as bronchitis and sinusitis, we focus on pneumonia and tuberculosis (TB).
NORMAL MATERNAL PULMONARY PHYSIOLOGY
In general, pregnant patients are deemed to be an at-risk population, primarily because of fetal considerations. However, pregnancy-induced adaptations to the immune system may also confer some degree of vulnerability to certain pathogens, although pregnancy is not traditionally considered a profound immunosuppressed state. Physiologically, maternal cellular immunity adapts to tolerate the presence of the fetus, with changes including decreased lymphocyte proliferative response, smaller number of helper T cells, decreased natural killer cell activity, reduced lymphocyte cytotoxic activity, as well as hormonally triggered inhibition of cell-mediated immunity.5,6 Humoral immunity is not compromised. In particular, pregnant patients seem to have an increased susceptibility to viral infections, which may be related to changes in innate interferon (IFN) responses during pregnancy.7,8
In addition to changes in immunity, there are significant adaptations of maternal pulmonary and systemic physiology. Table 9-1 describes the relevant anatomical and physiological changes seen in pregnant patients.9 Fetal metabolic demands on material circulation result in an increase in oxygen consumption by almost 20% during pregnancy. Increases in thoracic diameter and diaphragmatic excursion, despite restriction caused from the growing gravid uterus, allow pregnant patients to increase tidal volumes to meet the oxygen needs. Consequently, minute ventilation increases by almost 50%, without significant changes in respiratory rate. Pulmonary function tests show decreased functional residual capacity (decreased expiratory reserve volume and residual volume) but no impairment of inspiratory function.
TABLE 9-1 | Anatomical and Physiologic Changes of Respiratory System in Pregnancya |
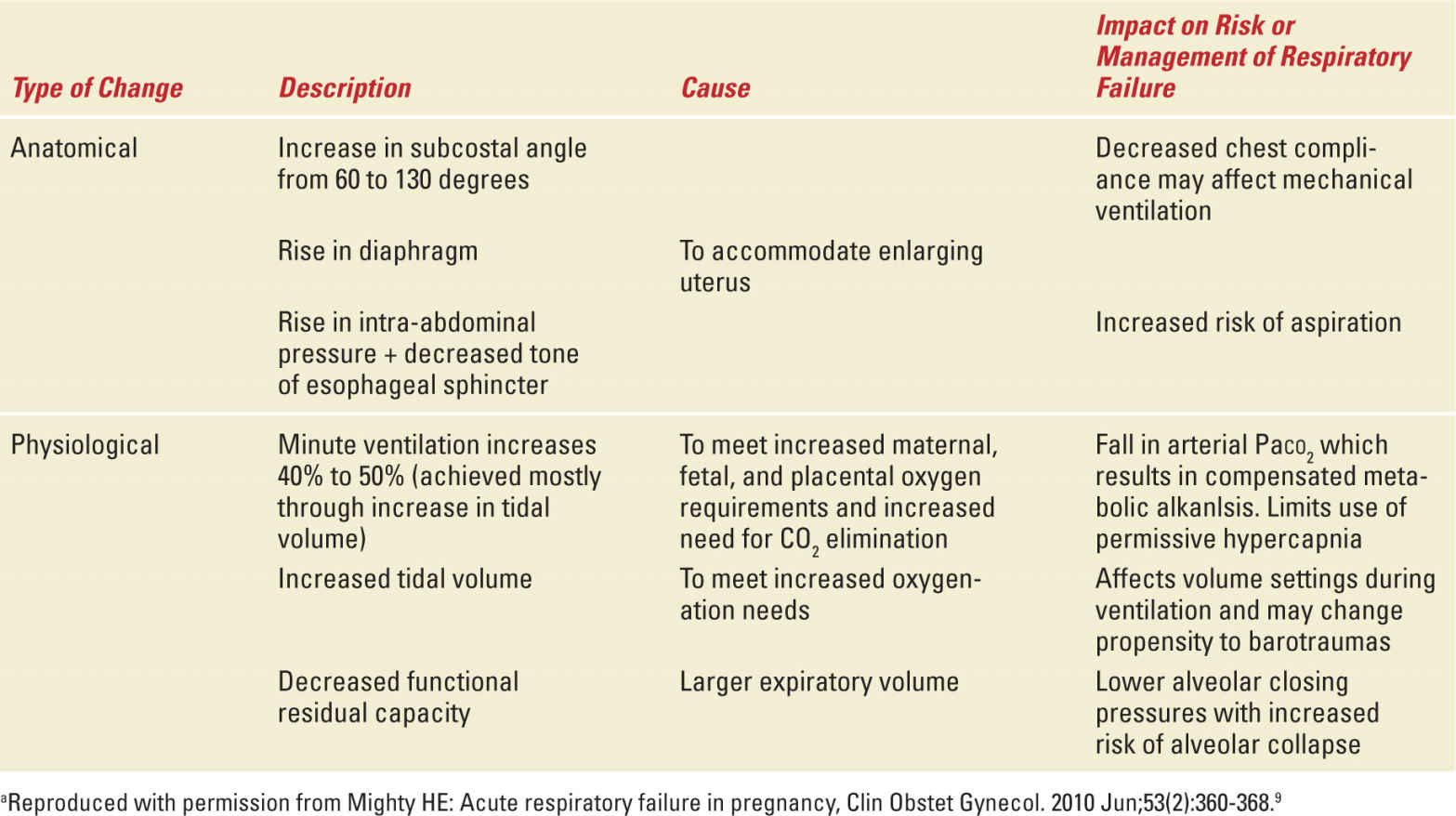
Oxygen and carbon dioxide levels also adjust to facilitate transfer of gases across the placenta to and from the fetus. Partial pressure of oxygen (Pao2) as measured in arterial blood gas is increased at 100 to 106 mmHg. Despite these higher levels, the increased oxygen consumption and decreased oxygen stores from lower functional residual capacity lead to a higher susceptibility to developing hypoxemia in times of distress.10 Hyperventilation to offset increased carbon dioxide production results in partial pressures of carbon dioxide at 26 to 32 mmHg. This chronic respiratory alkalosis is accompanied by an increase in bicarbonate renal clearance leading to coexistence of a metabolic acidosis and respiratory alkalosis.
CLINICAL PRESENTATION AND INITIAL WORK-UP
The clinical presentation of a pulmonary infection is similar to nonobstetric patients; symptoms of fever, chills, rigors, cough, sputum production, wheezing, pleuritic chest pain, and shortness of breath are often reported. Pregnant women may have subjective dyspnea at baseline but typically do not have tachypnea or respiratory distress. If present, this should raise concern for a pulmonary process or systemic condition warranting immediate attention. The differential diagnosis of the aforementioned presentation is broad and includes pulmonary edema, often seen in preeclampsia cases or triggered by tocolytic agents, asthma, pulmonary embolism or amniotic fluid embolism, as well as acute respiratory distress syndrome (ARDS) triggered by a host of conditions that may or may not be associated with pregnancy.9 Consequently, a targeted but thorough work-up is necessary to determine if an infection is present, especially for those patients with risk factors for pneumonia. Risk factors for the development of pneumonia include chronic respiratory conditions such as asthma, anemia, human immunodeficiency virus (HIV) infection, smoking, illicit drug use, and use of corticosteroids and other immunosupressants.11–15
Suspicion of severe respiratory infection should not be excluded with a negative radiograph as abnormal findings may be present in only 40% of cases.4
OVERALL MANAGEMENT AND ADDITIONAL CONSIDERATIONS
Management goals for the hospitalized obstetric patient with pneumonia include adequate oxygenation, fluid resuscitation to avoid hypovolemia-associated hypotension, and rapid administration of appropriate antibiotic therapy in a setting equipped to monitor for signs of deterioration in the mother and fetus. The critically ill obstetric patient warrants a multidisciplinary approach from critical care, maternal-fetal medicine, and neonatology to carry a critically ill complex patient to a gestational age of viability.16
The American Thoracic Society and the Infectious Diseases Society of America published guidelines for the management of community-acquired pneumonia (CAP) in adults in 2007; they serve as a starting point for obstetric patients.17 These guidelines discuss the decision-making process of treatment locations for a symptomatic patient, namely, in-hospital or outpatient settings. Intensive care unit (ICU) admission is recommended for those with severe CAP. Although only 10% of patients hospitalized for CAP require the ICU, these guidelines have not been prospectively validated for a pregnant population. Physiologically, pregnant women are more susceptible to hypoxemia, which can have rapid ill effects on both mother and fetus. As such, the threshold for both hospital admission and ICU transfer should be lower in this population.
Oxygenation and ventilation have specific management strategies in light of the changes in respiratory physiology in both the critically ill and noncritically ill pregnant patient with pneumonia.18 The oxygenation goal for patients should be greater than 90% by pulse oximetry or a Pao2 of 65 mmHg or higher, as fetal oxygenation may decrease at levels lower than this point. In cases warranting mechanical ventilation for acute respiratory failure and ARDS, the goal Pao2 is higher than the recommended Pao2 range of 55 to 80 mmHg for the nonobstetric patient. The mild chronic respiratory alkalosis should be maintained and overventilation avoided. As these patients hyperventilate at baseline to compensate for increased metabolism, they lack the buffering ability to counter a metabolic acidosis triggered by infection and can have a quick drop in pH. This must be balanced with prevention of excess respiratory alkalosis, which can cause constriction of the uterine blood supply. Permissive hypercapnia used in more severe cases of respiratory failure in the general patient population can be used in pregnant patients as long as concurrent fetal monitoring does not demonstrate signs of fetal acidosis in the form of repetitive late decelerations.19
Volume resuscitation and avoidance of hypotension are particularly important in this population as both mother and fetus are at risk for decreased organ perfusion. As maternal-fetal adaptations lead to vasodilation, high cardiac output, and increased intravascular plasma volume with associated lower blood pressure readings, the signs of developing sepsis can be easily missed.19 Crystalloid volume resuscitation with vasopressor support as needed should be used to support a pregnant patient through severe sepsis and shock, with close fetal monitoring throughout resuscitation and further management. Hypotension can also be worsened by aortocaval compression and decreased venous return; left lateral positioning should be used to displace the gravid uterus.
The third component is early and appropriate empiric broad-spectrum antibiotics administered within 4 to 8 hours of presentation.17,20,21 In general, beta-lactam antibiotics, certain macrolides, aminoglycosides, and most antivirals are considered safe in pregnancy. The next section describes the etiology of pneumonia and associated treatment strategies.
ETIOLOGY AND SPECIFIC TREATMENT
Pneumonia is a common cause of respiratory distress in the pregnant patient with rates estimated as high as 2.7 per 1000 deliveries.13 The etiology of acute respiratory infections in pregnant and postpartum women is similar to that of the general population, with Streptococcus pneumoniae and the atypical pneumonias being very common causes of pneumonia. True assessment of pathogen incidence in the literature is limited by variable diagnostic testing of patient cases. Table 9-2 lists pathogens in decreasing order of frequency.5 The 2009 H1N1 influenza epidemic raised awareness of the role of viral infections in causing severe pneumonia, both as primary events and with bacterial superinfection. In addition, with concerns of immune system modulation during pregnancy, a clinician must also consider more unusual culprits including varicella, fungi, and TB. This section highlights a selection of pathogen-specific pneumonias, with TB discussed in detail in the second half of the chapter. Treatments of choice are typically similar to treatment in the nonpregnant population.
Bacteriology of Pneumonia in Pregnancy (in Decreasing Order of Frequency)a |
aData from Khan S, Niederman MS. Pneumonia in the pregnant patient. In: Rosene-Montela K, Bourjeily G, Editors. Pulmonary problems in pregnancy. New York (NY):Humana Press; 2009. P. 177-96.
Bacterial Pneumonia
Bacterial pathogens are the most common cause of pneumonia in obstetric patients and present with similar clinical and imaging findings to the general population. S. pneumoniae is the most frequently isolated pathogen on bacterial cultures; with a rising proportion of drug-resistant strains.17 Other isolates include Hemophilus influenzae and the atypical pathogens, including Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella species. In patients with pre-existing bronchopulmonary disease such as asthma, chronic steroid or antibiotic use, and/or frequent hospitalizations, the differential must also include Moraxella catarrhalis, Pseudomonas aeruginosa, and Enterobacteriaceae species. Staphylococcus aureus is a particularly virulent pathogen, with both methicillin sensitive and resistant strains (MRSA) causing significant morbidity. The Panton-Valentin leukocidin toxin is associated with both strains and can cause high mortality even in immunocompetent hosts.22,23 S. aureus should be considered in patients with suspected or confirmed influenza virus in whom there is concern for a superimposed bacterial infection.
The diagnosis of a bacterial pneumonia is often made clinically based on symptoms and radiographic findings of a lobar infiltrate. The most recent guidelines do not recommend routine microbiological testing for outpatients with CAP unless the patient has risk factors for unusual pathogens, evidence for more severe disease, or presence of comorbidities.17 There are no specific recommendations for the pregnant patient, especially as treatment is generally well tolerated. However, in the pregnant patient with associated acute respiratory decompensation and critical illness requiring mechanical ventilation, swiftness and accuracy of pathogen identification is vital to minimize morbidity and mortality.
In general, antibiotic choices are similar to those for the nonobstetric patient population except for exclusion of tetracyclines and fluoroquinolones because of the theoretical risks of teratogenicity. Pneumonia in the outpatient population can safely be treated with azithromycin. For hospitalized patients, current recommendations are for dual agent therapy with both azithromycin and cephalosporin such as ceftriaxone.17 The regimen is adjusted and expanded based on the patient’s immune status, comorbidities, and risk factors. For instance, when community-acquired MRSA is a concern, vancomycin or linezolid may be added for the critically ill patient because of local resistance patterns to alternative agents.17 Vancomycin and linezolid may be used during pregnancy if required.
Viral Pneumonia
The most common viruses that cause pneumonia in the pregnant population are influenza and varicella-zoster virus (VZV). Both convey significant morbidity and mortality, although influenza A has recently become a pressing concern after the 2009 H1N1 influenza pandemic leading to poorer outcomes among pregnant patients with 7- to 13-fold higher risk of ICU admission and mechanical ventilation, higher rates of systemic complications such as myocarditis and ARDS, and severe neonatal outcomes.12,13,24–26 Influenza has been associated with a fivefold increase in hospitalization, cardiopulmonary complications, or death.27
The diagnosis of influenza is commonly made clinically and can be confirmed through testing of a nasopharyngeal or oropharyngeal swab of respiratory secretions. Commercially available Food and Drug Administration-approved rapid influenza diagnostic testing assays have variable ability to detect influenza depending on both virus type and viral load. When compared with up to 99% sensitive and specific real-time reverse transcription-polymerase chain reaction (PCR) testing, these rapid tests had only a 40% to 69% detection rate.28 Chest radiograph findings can reveal bilateral infiltrates or frank consolidation if a concurrent bacterial process is present. We recommend confirmation of the diagnosis with PCR when available.
Treatment should be initiated as soon as possible, even in the presence of a negative rapid test, given the high morbidity and mortality associated with influenza. As there is more safety data in pregnancy, the drug of choice is oseltamivir 75 mg twice daily for 5 days, with zanamivir 10 mg twice daily for 5 days as the second-line treatment.29 In very severe cases, the dose may be increased to 150 mg bid for a total of 10 days of treatment.
All viral pneumonias, even if only mild in severity, convey a risk of developing secondary superimposed bacterial pneumonia.30 In these situations, more focal consolidations can be evident on chest radiograph with persistent or recurrent respiratory and systemic symptoms. These patients should be managed with antibiotics targeted broadly at both viruses and bacteria, with more intensive management, as outcomes are worse for those with dual processes.31,32
Although much of this discussion has been focused on diagnosis and treatment in light of the worse outcomes in pregnant patients, the importance of prevention must be emphasized. The Centers for Disease Control and Prevention (CDC) recommends influenza vaccination in all pregnant patients, preferably as early as possible, using the inactivated vaccine.33 The vaccine has been shown to protect both the mother throughout pregnancy and postpartum period and the baby for its first 6 months of life.34–36 The inactivated flu vaccine is safe in all trimesters of pregnancy.
VZV is less commonly seen than influenza, with an incidence of 0.7 to 3 cases per 1000 pregnancies; in these, up to 20% may develop pneumonia. Mortality is higher among smokers and those with more than 100 skin lesions.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


