Tissue
Cell type
Uterus
Endometrium
Myometrium
Ovary
Luteinizing granulose
Preovulatory granulose
Corpus luteum
Other reproductive organs
Vagina
Testes
Breast
Ductal and lobular epithelial cells
Brain
Pituitary
Hypothalamus
Preoptic area
Others
Vascular endothelium
Thymus
Pancreatic islets
Osteoblast-like cells
Lung
2 The Mechanisms of the Cellular Action of Progesterone
Progesterone can evoke genomic or non-genomic responses upon its interaction with target cells. The term “genomic actions” refers to the cellular response involved in the activation of the genetic machinery, resulting in modulation of DNA expression. The genomic actions of progesterone are largely, but not only, mediated by the progesterone receptor (PR) [20]. The term “non–genomic actions” indicates the cellular responses to progesterone which involve alternative pathways, such as the activation of signal-transduction cascades, the generation of intracellular second messengers, and the modulation of protein kinases and ion fluxes (Fig. 1.1) [21, 22].
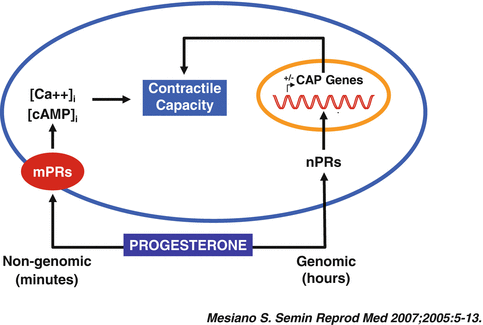

Fig. 1.1
Central paradigm for genomic and nongenomic progesterone actions on myometrial cells (Reproduced with permission from Thieme Publishers: Mesiano, S., Myometrial progesterone responsiveness. Semin. Reprod. Med., 2007. 25(1): p. 5–13 [149])
2.1 Genomic Actions of Progesterone and the Cytosolic Progesterone Receptor
The classical cytosolic PR [20], a member of the steroid/nuclear receptor superfamily, is a mediator of the genomic actions of progesterone. In resting conditions, this receptor is localized in the cytosol, within a large complex of proteins, including heat shock proteins and FK506-binding proteins, contributing to maintaining it in a transcriptionally inactive state (Fig. 1.2) [23].
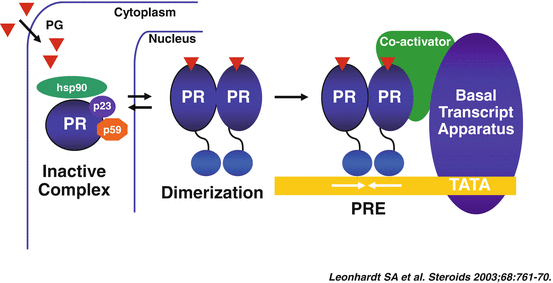

Fig. 1.2
Progesterone activation of the cytosolic progesterone receptor (Reproduced with permission from Elsevier: Leonhardt, S.A., Boonyaratanakornkit, V., Edwards, D.P. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids, 2003. 68(10–13): p. 761–770 [234])
This cytosolic receptor can be activated by ligand-dependent [23], and ligand-independent mechanisms [24]. In the ligand-dependent pathway, progesterone gains access into the cell through passive diffusion or facilitated transport, and binds to the receptor, which changes its conformation (including dimerization and shedding of the heat shock proteins) [25]. This process allows the dissociation of the PR from the chaperone complex, its translocation into the nucleus, and finally its interaction with the DNA, where binding in a homodimeric form to cis-acting DNA progesterone response elements (PREs) modulates the transcription of target genes [23]. The ligand-independent activation of the PR is, instead, the result of cross-talk between membrane receptors and intra-cellular kinases, including a cAMP-dependent kinase, the cyclin A/cyclin-dependent kinase-2 (Cdk2), the mitogen activating protein kinase (MAPK), the stress-activated p38 MAPK, and the protein kinases A and C [26].
The gene encoding the human PR is located on chromosome 11q22.1 and has eight exons. Alternative splicing allows for the synthesis of different isoforms of the receptor [27]. The two major isoforms of the PR are progesterone receptor-A (PR-A) and B (PR-B). These isoforms, although characterized by a different length, do not differ in their amino acid sequence: PR-B is 933 amino acids in length, while PR-A lacks 164 amino acids at the amino terminus. In vitro, PR-B is a stronger trans-activator than PR-A, whereas PR-A acts as a trans-repressor of PR-B and of other steroid receptors [28, 29]. Structurally, both isoforms consist of an amino-terminal region, a centrally-located DNA binding domain, and a carboxy-terminal hinge region containing nuclear localization signals, as well as the ligand-binding domain. Three transcription activation function (AF) domains have been identified within the PR amino acid chain. AF-1 is located upstream of the DNA binding domain, while AF-2 is located in the ligand-binding domain [30]. AF-3 is unique to the PR-B isoform, and is located within the N-terminal region [28, 31]. In addition, an inhibitory function region, located between the AF-1 and AF-3 domains, has been proposed to be responsible for the auto-inhibition and trans-repression of the PR [32]. Interestingly, most of the evolutionary changes in the human PR took place in this region [33]. (For more information on the structure of the Human Progesterone Receptor gene go to http://www.ncbi.nlm.nih.gov/gene/5241).
A third isoform of the PR, the PR-C, was also described [34–36]. This is a 60 kDa N-terminally truncated isoform, lacking the DNA binding domain, but containing the hormone binding region with the sequence for dimerization and nuclear localization [34, 35, 37]. The cytoplasmatic PR-C has been suggested to inhibit PR-B activity by sequestrating the locally available progesterone [37]. The nuclear PR-C can form heterodimers with PR-B, therefore interfering with its binding to the response elements in the DNA [37]. In contrast, PR-C can enhance the progestin-induced transcriptional activity of PR-A and PR-B isoforms, either by sequestrating the co-repressors and/or by increasing the capacity of the heterodimers of PR-A or PR-B with PR-C to recruit co-activators [35]. In this manner, PR-C could be involved in the modulation of the transcriptional activity of PR-A and PR-B, contributing to the pleiotropic effects of progestins [35]. Additional isoforms of the PR, such as PR-S [38] and PR-M [39] have also been identified and partially characterized. It has been proposed that the tissue responses to progesterone may be affected by changes in the expression ratio of the different isoforms [36].
Importantly, the validity of the immunoassay that was used in the identification of some of the PR isoforms, such as PR-C and PR-M, has been questioned [40, 41]. It has been demonstrated that the nuclear PR antibodies used may cross-react with cytoskeletal proteins (α-actinin, desmin and vimentin), suggesting that these antibodies are not specific for these PR isoforms [41].
2.2 The Role of Co-regulators in Progesterone Signaling
The activity of the nuclear PR is regulated not only by the hormone itself but also by co-regulators (co-activators and co-repressors) as well as by chromatin modifiers [42]. Co-regulators can enhance or inhibit gene transcription by creating a functional link between the ligand-activated receptors, the DNA and the transcription factors [43]. The existence of “intermediary factors” in the PR nuclear signaling was described more than four decades ago by the group of O’Malley [44]. Since then, the interest in co-regulators has increased, because of their possible involvement in the “transcriptional interference” in the tissue-specific responses evoked by nuclear receptors ligands, selective receptor modulators (i.e., Tamoxifen and Raloxifene), and their role in the pathogenesis/progression of neoplastic disease [45]. Thus, the possible involvement of progesterone co-regulators in the modulation of myometrial progesterone action should be taken into account [46, 47].
Progesterone co-activators include members of the “Steroid Receptor Co-activator” (SRC/p160) family [48], such as SRC-1, SRC-2, and SRC-3, which share a strong sequence homology [48, 49]. An involvement of these progesterone co-activators in normal growth, puberty, and female reproductive function, as well as in mammary gland development, is supported by studies on genetically-modified animals. SRC-1 [50, 51] is an important co-activator in the uterus, whereas SRC-3 is in the mammary gland [51, 52], and SRC-2 in both organs [46, 47]. Of note, SRC-1 and SRC-2 knockout mice manifested a deficient uterine response to progesterone stimulation. However, SRC-1 knockout mice preserved their fertility [50], whereas SRC-2 knockout mice had an early block of embryo implantation [46]. Progesterone receptor co-activators share a NRbox (also called LXXLL motif) that is necessary for binding to the “co-activator binding groove” in the receptor [49, 53].
Co-repressors of the progesterone receptors inhibit transcription factors recruitment and down-regulate the receptor-dependent gene expression. This is accomplished preferentially by recruiting histone deacetylases [54], which enhance tight nucleosome-DNA interactions and increase chromatin compaction [49]. However, the molecular basis of the interactions between steroid receptors and co-repressors is not well-defined [49].
2.3 Non-genomic Actions of Progesterone
The identification of steroid receptors on cells lacking a functional nucleus (i.e., spermatozoa, erythrocytes and platelets) supports the hypothesis of the existence of non-genomic steroid actions. This is a fast-track response system, and in contrast to the long response time (i.e., hours/days) of the “genomic” pathways, the cellular responses evoked by the “non-genomic” pathways are rapid [21, 22]. The first evidence in support of the existence of non-genomic progesterone actions came from the study of progesterone responses in germ cells such as oocytes and spermatocytes. Some of the non-genomic actions exerted by progesterone on these cells include changes in intracellular calcium concentrations [55–58], promotion of Na+ [59] and Cl− [60] fluxes, inhibition of adenylate cyclase activity with a consequent decrease in intracellular cAMP levels [61], and the involvement in G proteins-phospholipase C-inositol trisphosphate, and diacylglycerol signaling [62, 63].
“Membrane-initiated steroid signaling” defines the non-genomic activities of progesterone that are secondary to activation of membrane-localized progesterone receptors [21, 22]. Evidence that at least some of the non-genomic actions of progesterone are mediated by membrane located receptors include: 1) progesterone application outside the cell is more effective in decreasing intracellular cAMP concentrations than upon its cytoplasmic microinjection [64]; 2) progesterone activity is sustained after conjugation with synthetic polymers [65, 66] or its covalent binding to large molecules, such as albumin [62, 67], which prevent progesterone access into the cytosol; 3) progesterone effects are reduced in the presence of antibodies directed toward progesterone membrane-binding proteins [58]; and 4) the non-genomic activities of progesterone, such as Ca2+ influx, are not affected by inhibitors of genomic progesterone responses, including RU38486 and RU486 [55–57].
Progesterone high-affinity binding proteins and receptors have been identified on the cellular membranes of a variety of cells such as spermatozoa [55, 57, 58], porcine liver microsomes [68], porcine vascular muscle cells [69], as well as the brains of female mice knocked out for the classical PR [70]. Some researchers have previously argued that the existence of a progesterone binding site does not necessarily indicate that the receptor is functionally active in terms of cellular signaling, and that a characterization of non-classical receptors is still required [21, 22].
Non-genomic progesterone receptors display different affinities, binding capacities, and dose response/competition curves for progesterone and other molecules sharing progestin structure. For example, the recombinant human mPRγ, produced in an E. coli expression system, has a high affinity, saturable, single binding site for progesterone and several of its hydroxylated derivatives; however, recombinant human mPRγ does not bind and has no affinity for synthetic progestins and anti-progestins [71]. Similarly, there is evidence indicating the presence of at least two distinct membrane surface progesterone receptors in capacitated human spermatozoa: a high affinity site that is specific for progesterone, and a low affinity site that binds with equal affinity to 11β-hydroxyprogesterone and 17α-hydroxyprogesterone [57].
3 The Physiologic Effects of Progesterone
3.1 The Effect of Progesterone on the Immune System
The effect of progesterone on the immune system deserves special attention. The immune system in the female reproductive tract faces two opposing challenges: the consistent exposure to infectious pathogens, and in contrast, the need to be tolerant to both the allogenic spermatozoa and the semi-allogenic fetus. To overcome these challenges, the female sex steroids (i.e., estrogen and progesterone) control the function of the innate and adaptive immune systems in the reproductive tract according to the changes along the menstrual cycle and during pregnancy [72–74]. Indeed, in the rat uterus, major histocompatibility complex (MHC) class II positive cells, macrophages, granulocytes and dendritic cells were more abundant in the endometrial stroma and around uterine glandular epithelium in the estrus stages of the menstrual cycle relative to the diestrus stages in which progesterone is the dominant hormone [75]. Moreover, ovariectomy in mice results in a decrease in the number of uterine macrophages that can be restored by hormonal treatment [76].
The uterine/decidual natural killer (NK) cells are affected by progesterone as well: these cells have a role in promoting blastocyst implantation and maintenance of pregnancy [77, 78]. During the mid-late luteal phase the numbers of this unique population of NK cells is elevated [79, 80] as a result of the increased decidual concentrations of interleukin (IL)-15, and IL-15 mRNA [81]. Their number further increases during early stages of pregnancy and decrease from mid-gestation to term [79]. The immunologic recognition of pregnancy also leads to a higher expression of PR on membrane of uterine NK cells [82] and decreased cytotoxic activity in comparison to the non-pregnant state [83].
Progesterone diverts the T cell response toward Th-2 rather than Th-1, leading to higher secretion of IL-6 and IL-10, as well as supporting B-cell antibody production [84–86]. During pregnancy there is also a change in the antibody population and a shift toward non-symmetric “blocking” antibodies (those glycosilated by mannose-rich oligosaccharide only on one of the Fab regions and although can combine with antigen, they poorly activate phagocytosis, complement fixation, and cytotoxicity) and their prevalence increases from 9 % in the non-pregnant state to 29 % in pregnant women [87]. Yet, they can compete with symmetric and competent antibodies that have the same specificity and prevent their action. This was suggested as a mechanism to reduce the antibodies’ mediated response against the invading trophoblast during pregnancy and to control the equilibrium of maternal anti-fetal immune responses [88].
It has been reported that the effects of progesterone on the T cell response, B cell activity, generation of non-symmetric antibodies, and NK cytotoxicity is mediated by progesterone induced blocking factor (PIBF) (Fig. 1.3), a 34 kDa immunoregulatory protein synthesized by PR positive lymphocytes and CD56+ decidual cells [89]. The actions of PIBF include: 1) enhancement of the production of asymmetric antibodies [90]; 2) bias the T helper response toward Th-2 activity, resulting in increased concentrations of IL-3, IL-4 and IL-10 as well as decreased IL-12 production [91]; 3) the latter combined with the inhibition of perforin secretion by PIBF in a dose-dependent manner reduces the cytotoxic activity of NK cells [91, 92]. In summary, progesterone affects all arms of the immune system and propagates the maternal tolerance to the semi-allogeneic fetus.
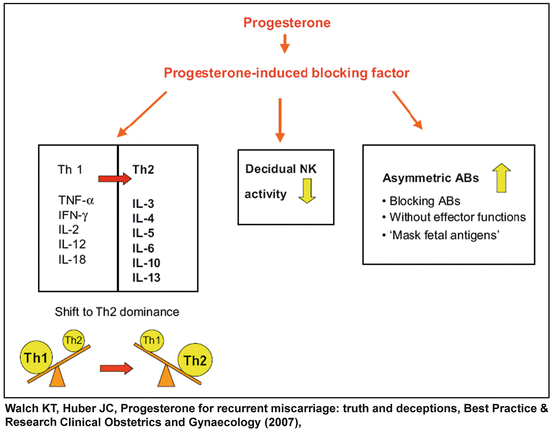

Fig. 1.3
The effect of progesterone and progesterone induced blocking factor (PIBF) on maternal immune system during pregnancy (Reproduced with permission from Elsevier: Walch, K.T. and J.C. Huber, Progesterone for recurrent miscarriage: truth and deceptions. Best Pract Res Clin Obstet Gynaecol, 2008. 22(2): p. 375–389 [75])
3.2 The Role of Progesterone in Non-pregnant Women
3.2.1 Progesterone and the Menstrual Cycle
Progesterone participates in control of ovulation, preparation and stabilization of the endometrium before implantation, regulation of the implantation process and maintenance of pregnancy [93]. During the follicular phase of the menstrual cycle, estrogen predominates and has a major role in the proliferation of the endometrium while progesterone concentrations are relatively low. Progesterone predominates during the secretory phase (maximal concentrations in the mid-luteal phase), inhibits the endometrial proliferation induced by estrogen, and changes the endometrial morphology to the secretory type [94]. However, the glandular and vascular elements continue to grow, resulting in progressive tortuosity [94]. Progesterone stimulates glycogen vacuole formation within glandular cells, resulting in the active secretion of glycoproteins and peptides by the glands into the endometrial cavity, as well as edema of the endometrial stromal tissue [95]. In the mid-luteal phase progesterone is responsible for the transformation of stromal cells into decidual cells, which is critical for the establishment of pregnancy. In the absence of pregnancy, the demise of the corpus luteum exerts a physiological progesterone withdrawal resulting in menstruation [93].
Previous exposure to estrogen is essential to stimulate synthesis of PR in endometrial cells. This enables progesterone to exert its anti-estrogenic effect on the endometrium [96] through several proposed potential mechanisms, such as down regulation of estrogen receptor expression [97], conversion of estradiol to a less active form (estrone sulphate) via the stimulation of 17-hydroxysteroid dehydrogenase and sulfotransferase [98], and suppression of estrogen-mediated synthesis/secretion of specific proteins (e.g., transcription of the proto-oncogene c-fos mRNA) [99].
In addition, progesterone increases the expression of tissue factor (TF) and plasminogen activator inhibitor-1 during decidualization [100]. It has been suggested that an increase in decidual TF concentrations is needed to secure rapid hemostasis during blastocyst implantation and placentation as well as the controlling of postpartum hemorrhage [100]. The association between the decidual expression of TF and progesterone was established by the differences in TF expression in confluent stromal cell cultures derived from proliferative phase endometrium. Stromal cell cultures that were treated with mifepristone did not increase their TF expression; moreover, administration of mifepristone to cell cultures previously exposed to estradiol + MPA or estradiol + progesterone decreased their TF content and TF mRNA expression [101]. Therefore, a low progesterone concentration could contribute to less effective decidual hemostasis, which may lead to increased decidual bleeding and a subsequent spontaneous abortion or preterm delivery.
3.2.2 Progesterone and the Myometrium in the Non-pregnant Uterus
Uterine contractile activity throughout the menstrual cycle is partially regulated by estrogen and progesterone [102–104]. This has been proposed to be mediated by cyclic changes in estrogen and progesterone receptor expression in the endometrium and sub-endometrium [105]. The decrease in the progesterone concentration in the transition from the luteal phase of one menstrual cycle to the follicular phase of the subsequent cycle is followed by increased uterine contractility, which aids in clearing menstrual contents [104]. The rise in estrogen concentration during the late follicular phase further increases uterine contractility, preparing the uterus to facilitate sperm motility toward the fallopian tube [104]. During the luteal phase, following an increase in progesterone concentration, the uterus is relatively quiescent [102, 104].
Of note, studies in non-pregnant women demonstrated that plasma progesterone concentrations do not reflect the actual progesterone concentrations in the myometrium. Akerlund et al. [106] measured the estrogen and progesterone concentrations in non-pregnant uteri of women with normal menstrual cycles, and demonstrated that there is no correlation between plasma and tissue progesterone concentration in the same individual, although the progesterone concentrations in the plasma and myometrial tissue change during the menstrual cycle. The authors reported that in peri- and postmenopausal women, the myometrial concentration of progesterone remains comparable to those in menstruating women, despite a substantial decline in plasma concentrations of this hormone, suggesting that the myometrial uptake of ovarian hormones may be saturated even if plasma concentrations are relatively low. Moreover, the myometrial/plasma ratio of progesterone decreases significantly during the luteal phase [106]. This may be due to down-regulation of the myometrial progesterone receptors following accumulation of progesterone in the myometrium [107].
3.2.3 The Effect of Progesterone on the Uterine Cervix During the Menstrual Cycle
The uterine cervix is a primary end organ that is responsive to pubertal hormonal action [108], cyclical changes in sex hormones during the menstrual cycle, pregnancy, labor and menopause [109–111]. The expression of PR changes significantly in the glandular epithelium of the cervix, reaching its peak in the early secretory phase and declining sharply afterwards [112]. Progesterone has a dramatic effect on the constituents of the cervical mucus, and hence, on the function of cervical secretions [113, 114]. The cervical mucus was defined by Odeblad et al. [113–115] as type E (estrogenic) and type G (progestogenic), the latter a thick and sticky mucus. Indeed, one of the suggested mechanisms by which progestins exert their contraceptive effect is through changes in the chemical properties of mucus [116, 117].
The available data suggest that progesterone has an effect on the cervix in the non-pregnant state; however, how and to what extent this effect is important in physiologic and pathologic conditions has yet to be determined.
3.3 Progesterone and Pregnancy
3.3.1 The Role of Progesterone in the Maintenance of Normal Pregnancy and Parturition
Estrogen and progesterone play a central role in pregnancy [118]. The corpus luteum is the main source of progesterone until the seventh week of gestation, then the placenta takes over as the main source of progesterone between 7 and 9 weeks of gestation, a transition termed the “luteal-placental shift” [119, 120] (Fig. 1.4). Indeed, ovariectomy before 8 weeks of gestation results in abortion, but has no effect on the pregnancy if performed after 9 weeks of gestation [121]. Maternal plasma progesterone concentrations rise during pregnancy from 40 ng/ml in the first trimester to 160 ng/ml in the third trimester [120]. At term, the placenta produces approximately 250 mg of progesterone per day, of which 90 % is secreted to the maternal circulation and only 10 % into the fetal circulation. However, the fetal plasma progesterone concentration is sevenfold higher than the maternal, probably due to the differences in their volume of distribution [122]. There are contradictory reports concerning the changes in amniotic fluid progesterone concentration during pregnancy: while one group reported that progesterone increases during gestation [123], others found that amniotic fluid progesterone concentration is higher in the second trimester than at term [124]. Of interest, the process of parturition at term [125] or preterm [126] was not associated with significant changes in amniotic fluid progesterone concentrations.
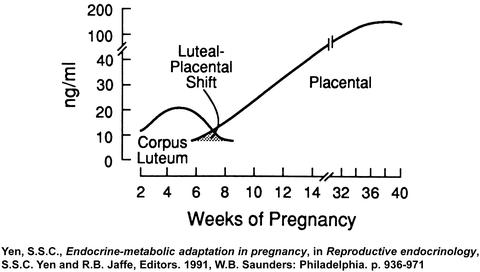

Fig. 1.4
The luteal-placental shift in progesterone production during pregnancy (Reproduced with permission from Elsevier Ltd.: Yen, S.S.C., Endocrine–metabolic adaptation in pregnancy, in Reproductive endocrinology, S.S.C. Yen and R.B. Jaffe, Editors. 1991, W.B. Saunders: Philadelphia. p. 936–971[235])
During pregnancy, progesterone is thought to maintain myometrial quiescence and inhibit cervical ripening, while estrogens have been implicated in increasing myometrial contractility and excitability, as well as in the induction of cervical ripening prior to the onset of labor [127–129]. However, before spontaneous parturition, the changes in sex steroid serum concentrations differ between different species. In many species, a fall in maternal serum progesterone concentration occurs prior to the onset of parturition, but the mechanism for this “progesterone withdrawal” depends, to a large extent, on whether or not the placenta or the corpus luteum is the main source of progesterone [130].
Luteolysis is a crucial component in the mechanism of parturition in the rat, mouse and rabbit [131–133]. An increase in local progesterone metabolism in both the uterus [134] and cervix [135] was associated with the onset of labor in mice. In sheep and goats, an increase in fetal plasma cortisol induces the placental production of P450 C17 enzymes (17α-hydroxylase and C17–20 lyases), which catalyze the conversion of progesterone to androstenedione, which is transformed into estrogen by aromatases [130]. In goats, the corpus luteum is responsible for most maternal progesterone production, and luteal regression is still required before parturition [133]. However, in primates (including humans) and guinea pigs there is no apparent change in circulating maternal progesterone concentration before parturition. The human placenta, in contrast to that of sheep, lacks P450 C17 enzymes, and therefore, cannot synthesize estrogen and androstenedione from C21-progestins, thus, progesterone is the final product of the human placenta.
A serum “progesterone withdrawal” was not demonstrated in humans or guinea pigs; yet, progesterone is considered important in pregnancy maintenance because inhibition of its action could result in parturition in both species. Administration of anti-progestins [i.e., mifepristone or onapristone] to pregnant women [136], primates [137] or guinea pigs [128] can induce abortion and/or labor [118]. Alternative mechanisms for the suspension of progesterone action without a serum progesterone withdrawal have been proposed, including: 1) binding of progesterone to a high affinity protein that reduces the functional active form [138]; 2) an increase in cortisol concentration during late pregnancy which may compete with progesterone binding to the glucocorticoid receptors, resulting in a functional progesterone withdrawal [139]; and 3) the conversion of progesterone to an inactive form within the target cell before interacting with its receptor. Indeed, human amnion and chorion can convert progesterone to the inactive 20α-dihydroxyprogesterone, and this metabolite increases with gestational age and around the time of parturition [140, 141]. However, none of these hypotheses have been proven [142]; therefore, the focus of investigation has shifted to the abundance and modulation of estrogen-progesterone receptor expression, and progesterone binding capability to its nuclear response element.
3.3.2 The Nuclear Progesterone Receptor in the Myometrium During Pregnancy and Parturition
Conflicting results have been reported regarding the role of the PR in the myometrium during human pregnancy and parturition [142–145]. These discrepancies have been partially attributed to the existence of multiple receptor isoforms, whose myometrial expression is spatially and temporally regulated throughout gestation [146]. Thus, it is likely that the results of the studies may be affected by the sampling site and the specificity of the assay. Of note, initial studies on PR expression in the human myometrium did not distinguish between the different isoforms and were performed on biopsies isolated from the lower uterine segment. In contrast, more recent studies tested the expression of the different receptor isoforms and focused preferentially on the fundal myometrium. The latter is more likely to reflect the molecular changes that mediate uterine contractility than the lower uterine segment, which reacts in favor of dilatation [36]. Finally, the non-genomic progesterone actions have broadened the research on the mechanism of labor toward identification of membrane progesterone receptors that may participate in the suspension of progesterone action.
The key mechanisms explaining the functional progesterone withdrawal include either a reduction of the total number of progesterone receptors within the target tissue, or a relative increase of inhibitory PR isoforms. Rezapour et al. [143] investigated the expression of progesterone receptors in the myometrium of women at term not in labor and in the active phase of spontaneous labor and found significant changes in the distribution of their receptors after the onset of labor. The active normal labor group had a higher receptor concentration in the upper uterine segment, as well as a higher upper to lower uterine segment receptor ratio than the not-in-labor group. Of interest, myometrial PR concentrations were lower in oxytocin-resistant labor than in normal labor. The authors suggested that although progesterone is involved in labor-associated changes in the myometrium through receptor mediated processes, it is not an inhibitor of myometrial contractility and, thus, not consistent with the progesterone withdrawal theory; however, this study did not discriminate between the different progesterone receptor isoforms. Supporting these findings are the in vitro reports that progesterone stimulates myometrial tonus and frequency of contractions [147], and has an anti-tachyphylactic effect on oxytocin-induced myometrial contractions [148].
In contrast, How et al. [144] supported the concept that a decrease in myometrial progesterone receptors may play a role in the onset of term and preterm parturition. Using myometrial samples from the lower uterine segment of women undergoing cesarean deliveries at term and preterm (in labor and not in labor), the authors found that in the pregnant myometrium, PR-A was relatively more abundant than PR-B. The intensity of immunostaining for progesterone receptors was lower in term than in preterm women not in labor and in preterm and term patients in labor than those not in labor. These local changes in myometrial PR expression with advancing gestation, and with labor, have been interpreted as the mechanism through which a functional progesterone withdrawal may occur, despite the absence of changes in the peripheral or myometrial concentrations of progesterone [144].
Pieber et al. [142] analyzed the labor-associated changes in the expression of PR-A and PR-B in myometrial samples obtained during term cesarean sections from women not in labor and in labor. While PR-A expression was detected only in the presence of effective labor, PR-B was equally expressed in labor and not in labor samples. Transient transfection of myometrial cells with PR-A and PR-B confirmed that the over-expression of PR-A has a dominant repressive effect on transcription of progesterone sensitive genes within human term myometrial cells. The authors interpreted that the expression of PR-B occurs throughout gestation and is required for pregnancy maintenance, whereas a higher expression of PR-A in the presence of effective labor at term may contribute to “functional progesterone withdrawal” [142]. However, in commenting on this study, Mesiano [149] noted that the immunoblotting data reported by Pieber et al. [142] demonstrated that the abundance of PR-B exceeds that of PR-A in laboring myometrium and that most of the in vitro studies showed that PR-A repression of PR-B transactivation occurs only when the PR-A/PR-B ratio is greater than one. Similar results were reported by Merlino et al. [150], which determined the PR-A and PR-B concentrations and their cellular localization in the lower uterine myometrium from preterm (not in labor) and term (not in labor and in labor) cesarean deliveries. The PR-A/PR-B ratio was significantly higher in term versus preterm myometrium; moreover, the ratio further increased in labor at term. The increase in the expression of the inhibitory PR-A [142, 151], and in the PR-A/PR-B ratio in human myometrium [150], was interpreted as the possible underlying mechanism of the “functional progesterone withdrawal”.
Mesiano et al. [152] demonstrated that the change in the PR-A/PR-B ratio occurs at the mRNA level. The authors compared the abundance of mRNAs encoding for PR-A and PR-B, as well as estrogen receptors (ER) α and β, in the lower uterine segment myometrium of women at term in labor and not in labor. The mRNA levels of ERα and of the homeobox gene HOXA10 were used as markers of progesterone responsiveness since their myometrial expression had been previously shown to be inhibited by progesterone. In laboring myometrium, the mean relative abundance of mRNAs encoding for PR-A, PR-B, and ERα was significantly increased than in non-laboring tissue, whereas ERβ was low and did not differ between the groups. There was a significant two to threefold increase in the PR-A/PR-B ratio in laboring compared with non-laboring specimens. Of interest, in non-laboring myometrium, the PR-A mRNA levels and the PR-A/PR-B mRNA ratio positively correlated with mRNA of ERα and HOXA10 in laboring myometrium. These positive correlations were interpreted as an indicator that progesterone responsiveness is inversely related to the PR-A/PR-B gene expression ratio and decreases at the onset of labor. Moreover, ERα could be an early gene, whereas HOXA10 a late gene responding respectively to changes in the PR-A/PR-B expression ratio. The positive correlation detected in non-laboring myometrium between ERα mRNA levels and those of contraction-associated genes, such as cyclooxygenase-2 (COX-2), and the oxytocin receptor, suggests that the process of human parturition is initiated within myometrial cells well before the onset of active labor [152].
Changes in the ratio of the PR isoforms during labor have also been reported in non-human primates. Haluska et al. [153] quantified the PR isoforms and PR mRNA expression in myometrium, decidua, and fetal membranes from rhesus monkeys during pregnancy and in spontaneous labor at term. No changes in the total PR expression were detected in the myometrium during pregnancy and labor, but there was a significant shift from PR-B dominance at mid-pregnancy to PR-A dominance in labor. In addition, both PR-A and PR-B isoforms and PR nuclear staining were nearly undetectable in amnion obtained during labor. The authors concluded that a shift to PR-A dominance in myometrium at term, together with a loss of PR in fetal membranes, may be the mechanisms for a “functional progesterone withdrawal” [153].
A possible role for prostaglandins (PGs) in the changes of PR isoforms during parturition has been suggested. Madsen et al. [154] proposed that the exposure to PGE2 and PGF2α, acting via the protein kinase C pathway, may facilitate functional progesterone withdrawal by increasing the myometrial PR-A/PR-B expression ratio. In addition, the membrane progesterone receptors (mPRs) in the human myometrium may also play a role in the mechanism of a functional progesterone withdrawal during labor [155].
3.3.3 The Membrane Progesterone Receptor During Pregnancy and Parturition
The first report on the existence of high affinity membrane-associated progesterone binding sites dates back to the study of Haukkamaa et al. [156] in 1984, on microsomes prepared from pregnant and non-pregnant uteri. Although at that time, the physiological function of these receptors was unknown, the study provided evidence that mPRs exist within the uterine tissue. It was noted that such receptors differ from their soluble cytosolic counterparts, previously identified in the human uterus, in terms of the specificity of their ligands.
Labor and sex-steroids differentially modulate the mPRs. Karteris et al. [155] reported the expression of two different functional mPRs (mPRα and mPRβ) in the myometrial cells of pregnant humans that are directly coupled to G-inhibitory proteins. This results in the inhibition of adenyl cyclase, a subsequent decline in cAMP concentrations and increased phosphorylation of the myosin light chain, which facilitates myometrial contractions. The authors proposed that, during labor, progesterone acts preferentially on its membrane receptors, a modus operandi that promotes the shift from quiescence to a contractile state. This change results from the altered PR-B/PR-A ratio, the changes in sex-steroids, and the existence of complex cross-talk between the nuclear and membrane progesterone receptors [155].
Fernandes et al. [157] combined bioinformatic analyses with the expression profile of mPRs to define their role in cycling human endometrium and gestational tissues. Sequence analysis suggested that these receptors belong to the “progestin and adiponectin receptors” family. The onset of parturition was associated with a marked reduction in myometrial mPRα and mPRβ transcripts. Of interest, the levels of mPRα expression were high in the placenta, and inversely correlated with that of the nuclear PR, indicating that mPRα may have an important functional role, particularly in reproductive tissues expressing low levels of nuclear PR [157].
3.3.4 Progesterone Oxytocin Responsiveness and Ca2+ Fluxes
Progesterone reduces the myometrial responsiveness to oxytocin through genomic [158] and non-genomic [159, 160] pathways. In 1929, Knaus [161] reported that “when an extract of the corpus luteum is injected into rabbits, the uteri of these animals subsequently suspended in physiological solution, fails to react to the oxytocic hormone of the posterior pituitary lobe”. This suggests that the corpus luteum extracts, described later as containing progesterone, induce uterine refractoriness for the uterotonic effect of oxytocin. After nearly nine decades, the exact mechanisms by which progesterone blunt uterine responsiveness to oxytocin is still not clear, and three potential mechanisms have been proposed: 1) progesterone represses the oxytocin receptor synthesis through its genomic action [158]; 2) direct interaction between progesterone and its metabolites with the oxytocin receptor [162, 163]; or 3) the continuous presence of intracellular high progesterone concentrations may alter the responsiveness of the oxytocin receptor through non-genomic effects [164].
The oxytocin receptor needs a cholesterol-rich microenvironment to become stable in its high-affinity state [165]. The intracellular binding of progesterone to the multi-drug-resistance P-glycoprotein interfere with cholesterol transport to and from the plasma membrane, and higher intracellular concentrations of progesterone inhibit cholesterol esterification as well [166], which reduces cholesterol concentrations in the plasma membrane [167]. Additionally, progesterone also increases the activity of 3-hydroxy-3-methylglutaryl (HMG-CoA) reductase, increasing the synthesis and membrane concentrations of the cholesterol precursors that are less active to support the high affinity oxytocin receptor [168]. The depletion of active membranous cholesterol forms leads to a low affinity mode of the oxytocin receptor,which may reduce its intracellular activity [169]. A decrease in the intracellular progesterone concentrations restores the cholesterol transport leading to an increase in active cholesterol concentration in the plasma membrane that supports the activity of high-affinity oxytocin receptor regaining its uterotonic effect [170].
Some of the activities of progesterone on myometrium may be mediated by its effects on the activity and the metabolism of cAMP [171] and inhibition of trans-membrane Ca2+ entry [172]. Treatment of human myometrial smooth muscle cells with MPA resulted in a significant reduction in oxytocin-mediated increase in intracellular Ca2+ concentration [172].
3.3.5 The Interplay Between NFκB and Progesterone in Pregnancy Maintenance and in the Onset of Labor
Nuclear factor kappa B (NFκB) is a transcription factor family classically associated with inflammation. Data indicate that myometrial NFκB activity changes with labor and its activation is regulated in a spatio-temporal fashion. It has been proposed that NFκB is an upstream regulator of multiple labor-associated processes, including the formation of contraction-associated proteins, inflammatory mediators (e.g., cytokines), uterotonic phospholipid metabolites (e.g., PGs), and the induction of extracellular matrix remodeling [173, 174]. The stimuli and mechanisms responsible for NFκB activation in spontaneous labor have not yet been elucidated. Increasing local concentrations of surfactant protein A [175], accumulation of advance glycation end-products [176], amnion cells mechanical stretch [177], and the paracrine or autocrine pro-inflammatory effects of the corticotrophin-releasing hormone [178] have been proposed as potential candidates.
NFκB activation favors the myometrial expression of inhibitory isoforms of the PR. Evidence in support of this includes: 1) a spatial correlation is suggested by the enhanced expression of PR-B and PR-C along with NFκB activation during labor, and these changes are selective for the fundal human myometrium [36]; 2) a temporal correlation has been proposed due to the correlation of PR isoform expression and local NFκB activation in the pregnant mouse uterus and in human fundal myometrium [36, 175]; 3) intra-amniotic injection of surfactant protein A to pregnant mice, which promotes uterine NFκB activation and preterm labor, as well as a rapid increase in uterine levels of PR-B and PR-C [36, 175]; 4) intra-amniotic injection of NFκB inhibitor (SN50) caused a decrease in the uterine levels of PR-B and PR-C [36, 175]; and 5) in vitro models demonstrated that the activation of the NFκB pathway in response to IL-1β treatment is associated with an increased expression of all three PR isoforms (PR-A, PR-B and PR-C) in myometrial cells [36].
The PR-mediated activation of target genes that modulate uterine contractility is antagonized by NFκB. Kalkhoven et al. [179] reported the existence of a mutual trans-repression between the PR and the RelA(p65) subunit of NFκB in different cell lines. This repression was independent from the PR isoforms and the cell type. The authors suggested that the most likely mechanism involved is a direct interaction between the two proteins that would result in an inactive heterodimeric complex on the DNA, which prevents co-factors and members of the basal transcriptional machinery to initiate transcription [179]. Other possible explanations for the mutual repression of RelA(p65) and PR include binding of these transcription factors to their respective cognate DNA elements, or competition for the same co-activators or transcription intermediary factors (transcriptional interference or squelching) [179]. A similar mutual negative interaction between NFκB and PR activity was reported in human amnion cells [180]. Stimulation of these cells with IL-1β resulted in NFκB activation that was followed by repression of progesterone-dependent transcription, even in the presence of excess PR [180]. This may be the case during spontaneous labor in humans: indeed, the constitutive activity of NFκB reported in human amnion cells in the presence of labor may contribute to the loss of myometrial quiescence, both by repressing the PR activity and increasing the expression of COX-2. The authors proposed that the increase in NFκB activity, near to, or at the time of labor, may represent a “watershed point at which labor becomes inevitable” [180].
The anti-inflammatory activity of progesterone may contribute to the prolongation of pregnancy by direct or indirect attenuation of the NFκB-mediated inflammatory cascade. Several observations support this view: 1) over-expression of the PR in amnion cells was associated with significant repression of NFκB reporter expression [180]; 2) the IL-1β induced up-regulation of COX-2 mRNA in immortalized human fundal myometrial cells was suppressed by exogenous administration of progesterone and associated with a rapid induction of the NFκB transactivation inhibitor, kBα [181]; 3) progesterone down-regulates cytokine production by human leukemia cell lines, mediated, at least in part, by suppression of NFκB activity [182]; and 4) physiological concentrations of progesterone suppress both the spontaneous and the IL-1(α and β)-mediated production of IL-8 by the uterine cervical fibroblasts in pregnant rabbits [183].
In contrast, Vidaeff et al. [184] demonstrated that pre-treatment of HeLa cells with progesterone before exposure to IL-1β resulted in a significant decrease in NFκB protein subunit p65 in the cytoplasm. However, it did not reduce the amount of nuclear p65 or affect the nuclear translocation of p65. The authors suggested that any possible role played by progesterone in preterm labor prevention is not exerted through anti-inflammatory mechanisms of NFκB down-regulation [184].
3.3.6 Changes in Myometrial Progesterone Co-regulators During Pregnancy
The possibility that changes in the activity of co-regulators can contribute to the functional progesterone withdrawal is currently an object of investigation. Condon et al. [185] proposed that a decline in the levels of PR co-activators in the pregnant uterus at term may antagonize PR function and contribute to the initiation of labor. Analysis of the mRNA and protein expression of PR co-activators in the fundal myometrium of 12 women in labor and 12 women not in labor revealed that laboring myometrium was associated with a lower mRNA and protein expression of SRC-2, SRC-3 and CBP (the cAMP-response element-binding protein) than non-laboring myometrial samples, while SRC-1 expression was relatively unchanged before and after the onset of labor.
Term gestation was associated with a decrease in the levels of histone H3 acetylation in the human and mouse uterus. Treatment of pregnant mice with trichostatin, a histone deacetylase inhibitor, delayed the onset of labor by 24–48 h. Altogether, these results suggested that a reduced uterine expression of progesterone co-activators at term would lead to a reduction in histone acetylation resulting in an impaired PR responsiveness and a functional progesterone withdrawal [185].
In 2005, a novel progesterone co-repressor, polypyrimidine tract-binding protein-associated splicing factor (PSF), was identified in the rat myometrium [186]: its mRNA expression increased as term approached and was up-regulated prior the onset of labor. PSF interferes with PR binding to its DNA response element, and enhances PR degradation. Within the human myometrium, PSF expression was significantly up-regulated as pregnancy progressed, particularly within the upper uterine region, and levels remained elevated in labor. Co-immunoprecipitations and DNA-binding assays showed that PSF directly interacts with nuclear PR and glucocorticoid receptor and specific co-regulatory proteins within the human myometrium [187]. These findings are suggestive of a role for myometrial PSF as a nuclear co-regulator and a potential contributor to functional progesterone withdrawal [186–188].
3.3.7 Progesterone Receptor in Fetal Membranes, Decidua, Placenta
There is evidence that all three isoforms, PR-A, PR-B and PR-C, are expressed in decidua and fetal membranes, yet there is still controversy concerning the predominant isoform [189–193]. A Western Blot analysis conducted by Goldman et al. [189] revealed that the major isoform in the human decidua is PR-B, whereas in the human amnion it is PR-C. In contrast, in a review on this subject, Taylor et al. [192] reported that immunohistochemical, Western blotting and real time RT-PCR techniques provide evidence that the major PR isoform in human decidua is PR-A, whereas in human term fetal membranes and syncytiotrophoblast it is PR-C [192].
The quantitative and qualitative expression of the PR isoforms in the decidua and fetal membranes may be subject to significant changes during labor [189, 190]. Our group reported that in fetal membranes obtained from women in labor, there is a PR-A predominance and a higher PR-A/PR-B ratio than in women not in labor, in which PR-B is the predominant isoform [190]. Similarly, Mesiano et al. [152] reported that mRNA encoding for the PR-A and PR-A/PR-B expression ratio increased significantly in the human myometrium at term in association with labor. Although the role of progesterone receptors in the fetal membranes has not yet been elucidated, it has been proposed that a shift in progesterone isoform expression may be part of a “feto-maternal signaling pathway in the initiation of labor” [191].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


