CHAPTER 2 Physiology of nerve injury and regeneration
Summary box
Introduction
In this chapter, we review the key physiological concepts underlying nerve injury and regeneration relevant to the clinical treatment of complex peripheral nerve disorders, such as brachial plexus palsies that manifest as paresis or paralysis of the upper extremity. Motor vehicle accidents cause approximately 70% of adult brachial plexus palsies (BPP).1 Young adult males comprise a significant proportion of patients suffering traumatic palsies, and they encounter substantial socioeconomic difficulty as a result of their disability.2–4 As the number of “extreme” sporting events and high-speed motor vehicle collisions increase, so does the worldwide prevalence of BPP.3,5–9 For countries such as Thailand, Vietnam, and India that rely on motorcycles as the main mode of transportation, the incidence of BPP is remarkably high. As the medical and surgical treatment of patients with BPP continues to improve, outcomes will be enhanced by increasing our knowledge of nerve and muscle pathophysiology after nerve injury and during neural regeneration.
Classifications of nerve injury
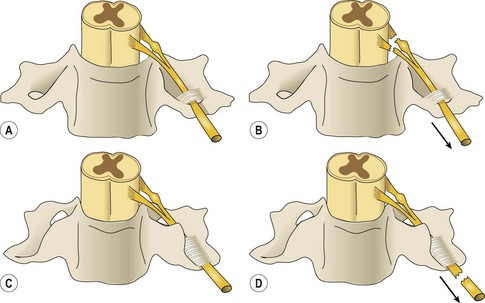
Injuries leading to brachial plexus palsies can be classified in several ways. They can be open or closed, sharp or ragged, clean or dirty. Consideration of the pathophysiology of these injury types led Dubuisson and Kline10 to propose an algorithm for the timing of nerve repair (Figure 2.1). Nerve injury can occur in either or all of the supraclavicular (roots, trunks), retroclavicular (divisions), and/or infraclavicular (cords, terminal branches) regions. Most injuries affect the nerve roots and trunks in the supraclavicular region. Supraclavicular injuries can be classified as preganglionic or postganglionic (Figure 2.2), but this seemingly simple classification has profound implications. In preganglionic lesions, the nerve roots are avulsed from the spinal cord, making nerve repair essentially impossible. In contrast, postganglionic lesions imply that the cell body is anatomically preserved so the nerve can be repaired with expectation of nerve regeneration.
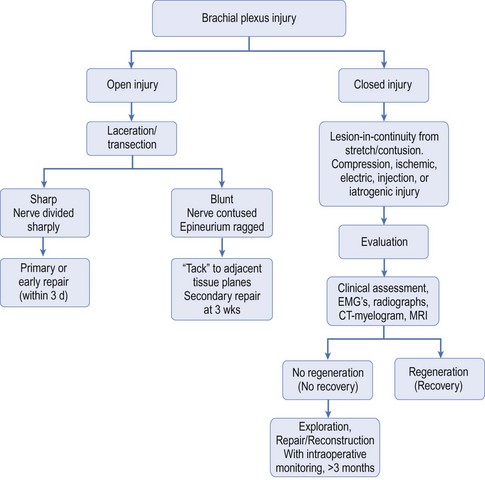
Figure 2.1 Algorithm for the timing of nerve surgery.
(Redrawn from Dubuisson A, Kline DG: Indications for peripheral nerve and brachial plexus surgery, Neurol Clin 10:935–951, 1992.)
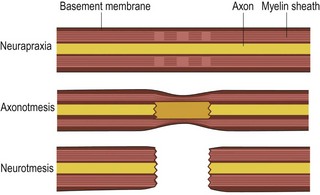
At the microscopic level, Seddon proposed a system for classifying nerve injury in 1943 that is still useful today.11 This classification system consists of neurapraxia, axonotmesis, and neurotmesis (Figure 2.3). Neurapraxia refers to segmental interruption of the myelin sheath, which leaves the axons and surrounding connective tissues intact; this type of injury recovers spontaneously within a few weeks. Axonotmesis refers to interruption of both the myelin sheath and the axons, but with sparing of the surrounding connective tissues (intact Schwann cell basal lamina); this injury may recover spontaneously within months to years if axonal regeneration is able to progress across the injury zone. Neurotmesis refers to interruption of all elements including the axons, myelin sheaths, and surrounding connective tissues; spontaneous recovery does not occur.
Reaction to nerve injury
Nerve cell response to nerve injury
The proximal segment is generally reduced in diameter due to loss of functional connectivity to the end-organ muscle and ensheathing Schwann cells. Consequently, the conduction velocity of the injured nerve is reduced. Microscopically, the degree of damage sustained by the proximal segment and neuronal cell body depends on the distance of the zone of injury from the cell body. If the zone of injury is far from the neuronal cell body, the Schwann cells degrade and the axonal degradation may extend just to the adjacent node of Ranvier. However, if the zone of injury is near or adjacent to the neuronal cell body, neuronal degeneration may extend all the way to the cell body to cause neuronal cell death. For example, apoptosis-related cell death in dorsal root ganglion neurons following axonotmesis can reach 50%.12 If the nerve cell body survives, stereotyped changes occur. The nucleus migrates to the periphery of the cell and select cytoplasmic elements (eg, Nissl granules, endoplasmic reticulum) undergo chromatolysis (Figure 2.4). Cell survival has been shown to rely upon the Schwann cells and trophic molecules present in the immediate environment.13,14
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree