Physiologic Considerations
Immanuela Ravé Moss
Successful transition of the respiratory and pulmonary circulatory system from the fetal to the neonatal state determines postnatal survival. The lungs of the full-term infant do not appear to be ready for postnatal gas exchange. The full complement of airways (but not alveoli) is developed, but the lungs are filled with fetal pulmonary fluid (30 mL/kg body weight).
Although shallow fetal breathing movements occur 30% of the time in the near-term fetus, they are distinctly different from postnatal breathing. As a result of intrauterine PO2 levels of 20 to 30 mm Hg, the fetal pulmonary circulation is constricted, pulmonary vascular resistance is high, and pulmonary blood flow is low. The compliance of the liquid-filled fetal lung at term is low and the compliance of the fetal chest is high.
Thus, major changes must occur rapidly and concurrently following birth so the lung can assume its air-exchanging function. Continuous breathing and ventilation must begin. Pulmonary functional residual capacity must be established. The pulmonary vasculature must dilate to permit an increase in pulmonary blood flow, and fetal pulmonary fluid must be absorbed. These processes are discussed in the following sections.
ONSET OF AIR BREATHING AT BIRTH
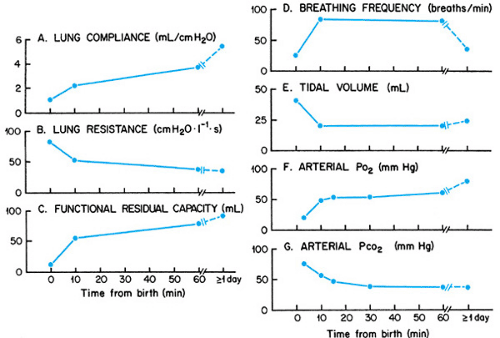
The onset of postnatal breathing is associated with an abrupt, birth-related increase in PCO2 and a decrease in PO2. These chemical inputs, together with a barrage of environmental stimuli including cooling, light, sound, touch, and pressure, promote arousal and powerful breathing efforts. With time, the initial, forceful, deep breaths diminish and are replaced with more moderate tidal breathing. Respiratory frequency shortly after birth is high. The high frequency is attributed to a reflex mediated by J receptors stimulated by the high interstitial pressure created by fetal lung fluid. As the fetal fluid is absorbed into pulmonary capillaries, breathing frequency gradually diminishes. Although the time course varies, stable respiratory function is usually attained by the end of the first postnatal day (Fig. 40.1).
The first breath is critical to the success of the transition from fetal to neonatal life. Forceful contraction of the inspiratory muscles generates the large subatmospheric transpulmonary pressures necessary to overcome surface and viscous forces and open air passages. The first expiration is also active and requires contraction of the expiratory muscles. Because vocal cord adduction occurs at the same time, the first expiration does not proceed to the prenatal lung volume. Instead, the forceful expiration against a partially closed glottis generates positive pressure in the airways and promotes the retention of some air in the lungs, thus initiating the establishment of gaseous functional residual capacity. The active inspiratory–expiratory sequence is repeated over subsequent breaths, so that with time, the air volume remaining in the lung at end-expiration increases until functional residual capacity is established (see Fig. 40.1).
The success of this process depends on adequate secretion of surfactant by alveolar type II cells, and on the establishment of an intraalveolar/acinar film, apparently in the form of stable bubbles, of surfactant-enriched material that lowers the surface tension of the lung.
The newly formed lung air–liquid interface modifies the geometry of the pulmonary vasculature, which, in combination with the increased PO2, recruits, distends, and opens pulmonary capillaries, dilates pulmonary vessels, and increases pulmonary blood flow. This vascular adaptation is crucial for successful absorption of fetal lung fluid and its removal by the pulmonary circulation.
The absorption of fetal lung fluid is promoted by a perinatal surge in epinephrine that activates beta-adrenoreceptors. These receptors, in turn, activate transepithelial sodium transport utilizing apical sodium channels and a basolateral sodium–potassium–adenosine triphosphatase pump. Of note, this process does not seem to depend on aquaporin-type water channels, although the number of these channels also increases around the time of birth.
Lung fluid absorption (and surfactant synthesis) is enhanced by a synergistic influence of thyroid and steroid hormones triggered by the abrupt increase in PO2 at birth. This absorption
is also favored by a pressure gradient from the airspaces to capillaries created by osmotic and hydrostatic forces aided by the positive airway pressure that is created by the expiration against the partially closed glottis. The progressive increase in air and decrease in fetal fluid gradually increase the compliance of the lung and enhance oxygenation. As a consequence, pulmonary resistance decreases further, and the match between lung ventilation and pulmonary perfusion improves (see Fig. 40.1).
is also favored by a pressure gradient from the airspaces to capillaries created by osmotic and hydrostatic forces aided by the positive airway pressure that is created by the expiration against the partially closed glottis. The progressive increase in air and decrease in fetal fluid gradually increase the compliance of the lung and enhance oxygenation. As a consequence, pulmonary resistance decreases further, and the match between lung ventilation and pulmonary perfusion improves (see Fig. 40.1).
RESPIRATORY MECHANICS, BREATHING DYNAMICS, AND GAS EXCHANGE IN THE NEONATE
Pulmonary gas exchange depends in part on the passive mechanical properties of the lung and chest wall. Total respiratory compliance (i.e., the change in lung volume per unit change in transpulmonary pressure) can be assessed in vivo with various techniques. Lung compliance can be measured in isolated lungs, and chest wall compliance is the difference between total respiratory compliance and lung compliance.
The chest wall of the neonate is very compliant because of the predominance of thin cartilage and poorly mineralized bone. Therefore, low respiratory total lung compliance in the newborn reflects poor compliance of the lung itself. Lung compliance is lower in the neonate than in the adult, while the stability of the newborn chest and thus its ability to recoil after an exhalation is relatively low. Static resting lung volume is determined by the balance between the tendency of the lung to collapse and the ability of chest wall recoil to prevent collapse. Poor lung compliance and poor chest recoil mean that transpulmonary pressure generated at equilibrium and resting lung volume are smaller, corrected for size, in neonates than in adults.
Dynamic compliance, which is measured during normal breathing and is influenced by the frequency of breathing, is lower, relative to adults, than static compliance. The reasons for the lowered dynamic compliance in the neonate are thought to be (a) the viscous properties of newborn lung tissue and (b) distortion of the pliable newborn chest, resulting in an unbalanced and unequal mechanical function of the chest wall.
During normal breathing, the dynamic functional residual capacity of the neonate is greater than what would be predicted from the passive balance between the inward recoil of the lung and the outward recoil of the chest wall. This variance occurs for two reasons: First, the relatively high breathing frequency in the neonate does not create sufficient time for full expiration. Second, persistent postinspiratory diaphragmatic contraction and laryngeal adduction during expiration hinder complete expiration. The resultant positive airway pressure favors the reabsorption of excess intrapulmonary fluid, helps to keep the airways open, and maintains a relatively large end-expiratory volume, enhancing gas exchange. This situation changes in active sleep, during which laryngeal adductor muscle activity is reduced, so that expiration is more complete and the volume expired is greater. This, in combination with decreased muscle tone and intercostal muscle activity, produces a lower end-expiratory lung volume and, thus, less optimal oxygenation during active sleep.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree