Maged M. Costantine, MD
HEMATOLOGIC AND COAGULATION SYSTEMS
INTRODUCTION
Pregnant women commonly use prescribed and over-the-counter medications with the average US and Canadian pregnant woman using more than two drugs during the course of their pregnancy. Almost one third of them used four or more drugs.1 One reason for this is that some women enter into pregnancy with preexisting medical conditions that require pharmacotherapy; and for many others, pregnancy itself can lead to medical conditions such as nausea, vomiting, and gestational diabetes that require treatment. Moreover, human pregnancy is characterized by profound anatomic and physiologic changes that affect virtually all systems and organs and induce profound alterations to the pharmacokinetic and pharmacodynamics properties of many medications. Many of these changes begin since early gestation. Whereas, some of these changes are secondary to hormonal changes in pregnancy, others are essential to support the growing fetus and the pregnant mother. Understanding pregnancy physiology is essential to the clinician because of the potential implications on pharmacotherapy during pregnancy. The goal of this chapter is to summarize some of the systems, physiologic changes during pregnancy that may affect medication pharmacokinetics.
CLINICAL CASE
A 30-year-old G1P0 at 28 weeks of gestation presents for prenatal care. Patient has a history of epilepsy, and takes phenytoin 600 milligrams daily. During the clinic visit, you noted slurred speech, ataxia, and nystagmus. You ordered phenytoin levels and found that her total phenytoin level was 17 mg/L (normal 10-20 mg/L), and her free phenytoin level was 2.6 mg/L (normal 1-2 mg/L). After decreasing the dose, the symptoms completely resolved. It appears that this patient had phenytoin toxicity. In pregnancy, because of the decrease in serum albumin, the amount of free or unbound phenytoin may increase leading to increased risks of toxicity. The latter reflects the importance of measuring free levels of medications with significant protein binding during pregnancy.
CARDIOVASCULAR SYSTEM
The cardiovascular system is one of the most affected systems as pregnancy leads to significant anatomic and physiologic changes. Myocardial contractility, compliance, and ventricular wall mass increase in normal pregnancy.2 Maternal cardiac output (CO) is increased by 30% to 50% because of increases in both heart rate and stroke volume.3 The majority of the increase occurs early in pregnancy such that by the end of the first trimester 75% of such increment has already occurred.4 At 28 to 32 weeks, CO plateaus and remains relatively stable until delivery.5 During the third trimester, the increase in heart rate becomes the main contributor to the increased CO. This increase in CO allows uterine blood flow to increase by 10-fold (17% of total CO compared with 2% prepregnancy) and renal blood flow to increase by 50%. Minimal changes occur on liver and brain blood flow.6 In addition, when compared with nulliparous women, multiparous women have higher CO (5.6 vs 5.2 L/min), stroke volume (73.5 vs 70.5 mL), and higher heart rates.7 As CO increases, pregnant women experience a significant decrease in both systemic and pulmonary vascular resistances.3 Secondary to the vasodilatory effects of progesterone, nitric oxide, and prostaglandins systemic vascular resistances decrease during early pregnancy reaching a nadir at 14 to 24 weeks. Subsequently, vascular resistances start rising progressively approaching the prepregnancy value at term.3 Blood pressure tends to fall toward the end of the first trimester and then rises again in the third trimester to prepregnancy levels.8 Therefore, physiologic hypotension may be present between weeks 14 and 24 secondary to the decrease in systemic vascular resistances. This is especially important in patients with chronic hypertension and who are on antihypertensive agents (Table 37-1).
Summary of Cardiovascular Changes During Pregnancy |
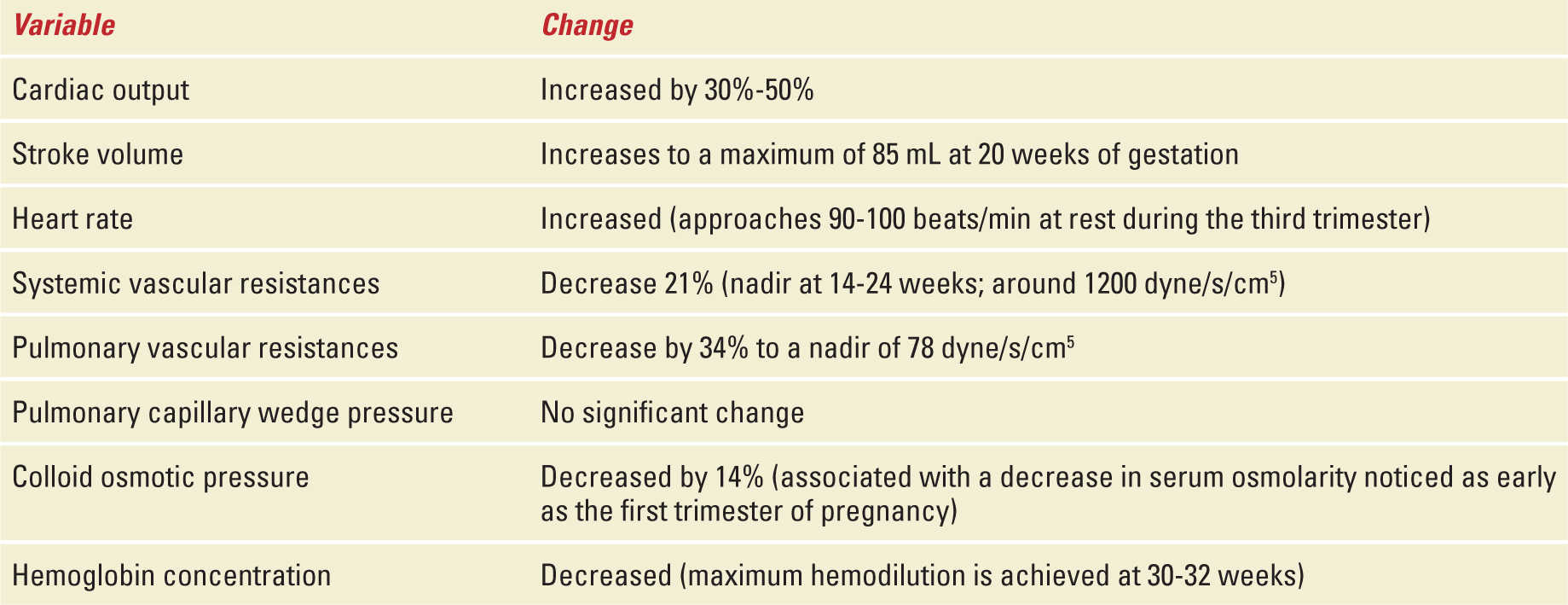
During pregnancy, maternal blood volume increases by 40% to 50% above nonpregnant values. The increase in effective volemia starts at as early as 6 to 8 weeks of gestation and approaches maximum values at 32 weeks.9 Despite the increase in blood volume, central venous and pulmonary artery occlusion pressures remain unchanged secondary to an increase in compliance of the right and left ventricles.10 In other words, the ventricles are able to accommodate the increased volume by increasing their volume maintaining normal filling pressures. The precise etiology of the increase in blood volume is not clearly understood; however, the mechanism may be through nitric oxide-mediated vasodilatation coupled with increased mineralocorticoid activity leading to water and sodium retention.11 This increase in blood volume provides the pregnant woman the capability to bleed close to 1 liter during delivery without compromising end organ perfusion.12 Production of arginine vasopressin (resulting in increased water absorption in the distal nephron) is also increased during pregnancy and thought to contribute to hypervolemia. Secondary hemodilutional anemia and a decrease in serum colloid osmotic pressure (because of a drop in albumin levels) ensue.
The increase in blood volume, capillary hydrostatic pressure, and water within the body increase significantly the volume of distribution of hydrophilic substances. For some drugs, a larger volume of distribution could necessitate a higher initial and maintenance dosage regimen to obtain therapeutic plasma concentrations. In addition, because of the decrease in serum albumin highly protein bound compounds may display higher free levels because of decreased protein binding availability. To put things into perspective, if a drug is 99% bound to albumin in nonpregnant patients but 98% bound to albumin in pregnant patients then the active fraction of the drug during pregnancy is effectively doubled. Digoxin, midazolam, and phenytoin are examples of such medications.
RESPIRATORY SYSTEM
The sharp increase in estrogen concentrations during pregnancy leads to increased vascularity and edema of the upper respiratory mucosa.13 These changes result in an increased prevalence of rhinitis and epistaxis in pregnant women. Theoretically, inhaled medications such as steroids used in the treatment of asthma could be more readily absorbed in the pregnant patient. Despite this theoretical concern, there is no evidence of increased toxicity with the use of these agents during pregnancy.
Mainly driven by progesterone, minute ventilation increases by 30% to 50% secondary to a 40% increase in tidal volume. Respiratory rate remains unchanged during pregnancy.14 The increase in ventilation results in an increase in the arterial partial pressure of oxygen (PaO2) to 101 to 105 mmHg and a diminished arterial partial pressure of carbon dioxide (PaCO2), with normal values of PaCO2 during pregnancy of 28 to 31 mmHg. This decrement allows for a gradient to exist between the PaCO2 of the fetus and the mother so that carbon dioxide can diffuse freely from the fetus into the mother through the placenta and then be eliminated through the maternal lungs.
The normal maternal arterial blood pH in pregnancy is between 7.4 and 7.45, consistent with a mild respiratory alkalosis. The latter is partially corrected by an increased renal excretion of bicarbonate, rendering the normal serum bicarbonate between 18 and 21 meq/L during gestation.15 As pregnancy progresses, the increased intra-abdominal pressure (likely secondary to uterine enlargement, bowel dilation, and fluid third spacing to the peritoneal cavity secondary to decreased colloid-osmotic pressure) displaces the diaphragm upward by 4 to 5 cm leading to alveolar collapse in the bases of the lungs. Bibasilar atelectasis results in a 10% to 20% decrease in the functional residual capacity and increased right to left vascular shunt.16,17 The decrease in expiratory reserve volumes is coupled with an increase in inspiratory reserve volumes, as a result no change is seen in the vital capacity.16
RENAL SYSTEM
The effects of progesterone and relaxin on smooth muscles are also seen in the urinary system leading to dilation of the urinary-collecting system with consequent urinary stasis, predisposing pregnant women to urinary tract infections.18 This is more common on the right side secondary to dextrorotation of the pregnant uterus and the right ovarian vein that crosses over the right ureter.
The 50% increase in renal blood flow during early pregnancy leads to a parallel increase in the glomerular filtration rate (GFR) of approximately 50%. This elevation in GFR is present as early as 14 weeks of pregnancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


