Pharmacologic Principles of Drug Therapy
George C. Rodgers Jr.
Nancy J. Matyunas
The primary goal of drug therapy is to cure or ameliorate disease while causing minimal side effects. To achieve this goal the most appropriate drug is chosen and used in an optimal manner. Often, the result of the improper use of a drug is either therapeutic failure or toxicity. These consequences are preventable if the principles of pharmacodynamics and pharmacokinetics are considered in the selection and monitoring of a treatment regimen. This chapter reviews these principles as they apply to the use of drugs in pediatric patients.
SCIENTIFIC BASIS OF PEDIATRIC CLINICAL PHARMACOLOGY
Decision making in pediatric drug therapy has historically been made on the basis of data gathered in adults, even though it is clear that developmental age directly influences the disposition of drugs and chemicals. Great variability in drug response exists even among preterm and term newborns, infants, toddlers, children and adolescents. These differences have led to such widely publicized problems as the “gray-baby syndrome” due to delayed chloramphenicol metabolism in neonates, severe jaundice from the use of sulfa drugs that displace bilirubin in newborns, and the “gasping syndrome” in newborns treated with drug preparations containing the preservative benzyl alcohol.
The recognized gold standard for evaluating clinical efficacy is the prospective, randomized, controlled trial. A relative lack of good clinical data in children exists because of the difficulties associated with performing such studies. However, the body of clinical drug data in children is growing, in part due to a federal mandate requiring drug manufacturers to provide pediatric drug-use guidelines. Drug manufacturers also must provide data to support the safe and effective use of drugs in children.
Some of the difficulties associated with performing drug studies in children include ethical issues, difficulty obtaining parental or patient consent, lack of pediatrician interest in performing clinical research, the small numbers of children qualifying for studies, and the lack of financial incentive for the pharmaceutical industry because of the limited market potential for most drugs in pediatric patients. Because drug response may be variable across age groups, these differences must be taken into consideration when designing clinical trials, thus adding further complexity to the study design. To gather adequate numbers of patients to ensure statistical power, trials often require the participation of multiple research centers. For similar reasons, few drugs are developed specifically for diseases primarily afflicting children.
In the absence of controlled, randomized clinical trials in children, pediatricians either must extrapolate information from adult studies or from uncontrolled clinical reports in children, both of which sources of data should be used cautiously. Reliance on adult studies may lead to either underdosing or overdosing. Uncontrolled clinical reports in children often can be misleading, because many factors unrelated to the use of the drug may affect outcome. Only a properly designed controlled trial effectively eliminates confounding variables. Pediatricians must bear in mind the uncertainty that the lack of data adds to their drug selection and exercise appropriate caution.
SELECTION OF AN APPROPRIATE DRUG
The selection of the appropriate drug is a four-step process. First, the therapeutic goal must be clearly defined, such as the treatment of infection or maintenance of blood pressure. Second, all of the potentially useful therapeutic agents must be identified. Third, the most appropriate agent must be selected based upon the patient’s age, developmental status, history of allergy or previous intolerance, drug resistance (in the case of antibiotics), and the availability and cost-effectiveness of the various agents. Fourth, the optimal dosing regimen and therapeutic monitoring plan must be determined.
For a few diseases, such as Gaucher disease, only one therapeutic option may be available. For most diseases however, several possible options exist. Some drugs should not be used because of age-specific adverse effects. For example, the adverse effect of tetracyclines on tooth development in children younger than 8 years dictates that these drugs generally should not be used in this age range. The lack of a U.S. Food and Drug Administration (FDA)—approved pediatric indication is not a legal prohibition against the use of the drug in children. It is always important to ask patients or family members about possible drug allergy or intolerance. Common, relatively benign effects, such as antibiotic-induced gastrointestinal discomfort, may be an acceptable side effect and should not preclude the use of the drug if absolutely necessary. On the other hand, if a serious reaction, such as an urticarial rash or respiratory difficulty is reported, the offending drug and its chemical analogues should not be used.
The soaring cost of drug therapy, due in part to the introduction of many innovative but expensive agents, has led to increased efforts to control this segment of health care expenditures. An aspect of disease management reviews the value of the drug in the overall management of a particular disease. A drug with a high unit cost may be a better value if it controls the disease more effectively or can be administered in a manner that improves adherence to therapy. Most health care facilities, managed care organizations, and state Medicaid programs use drug formularies that encourage the use of the most cost-effective drug. Formularies can be useful in providing the pediatrician with a reference guide of relative drug costs or cost effectiveness. There will always be patients whose condition requires the use of a drug not included on the formulary, and all programs using formularies provide some mechanism to obtain an alternative agent. Usually, it is to the patient’s and pediatrician’s advantage to select an agent on the formulary. Because some families may not be able to obtain the prescribed drug because of the high cost, pediatricians should include affordability when considering which drug to use. Increasingly, drug manufacturers provide patient assistance programs that provide maintenance drugs at no cost when the family does not have the resources to purchase them.
PHARMACODYNAMICS
Pharmacodynamics describes how the drug affects the body. It encompasses the mechanism of action, clinical effects measured, and the safety profile of the agent. Most drugs produce their therapeutic (and toxic) effects through interacting with receptors. Children may respond to a drug in a way different from that of an adult because they have increased or decreased numbers of receptors, altered receptor binding affinity for the drug, or the organ may not respond to receptor stimulation in the same fashion as an adult. The result of these alterations may be increased toxicity that precludes use of the drug, decreased clinical response that requires larger doses of the drug, or paradoxical effects. The paradoxical excitability in children caused by phenobarbital is an example of altered receptor response and is independent of the dose administered.
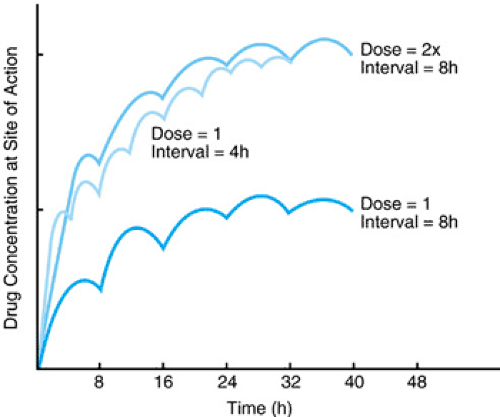
For most drugs, the concentration of unbound or free drug at its site of action is directly proportional to the pharmacologic response. It is assumed that the whole blood, plasma, or serum concentrations measured are proportional to tissue concentrations, and these are used as a surrogate. The manipulation of the dose and dosing interval significantly affects the concentration of the drug at its site of action. In general, a larger dose and more frequent administration produce higher drug concentrations at the site of action. Figure 7.1 shows how varying the dose and/or dosing interval produces very different drug concentrations. Thus, any change in the dose or dosing interval can result in a change in the drug concentration and potentially alter the efficacy or toxicity of the drug.
The dosage form of the drug also may dictate the choice of dose and dosing interval. To improve adherence to the drug regimen, many sustained-release preparations have been developed. These preparations usually allow for once or twice daily dosing and produce less variation in drug blood levels over the dosing interval. Switching between regular-release and sustained-release formulations usually requires an adjustment in both the dose and dosing interval, although the total daily dose remains the same. Failure to make these adjustments may result in undesirable blood concentrations.
Optimal dosing may be one of the greatest challenges facing pediatricians. Extrapolating a dose from adult studies may result in either underdosing or overdosing, leading to adverse effects. If a dose must be extrapolated from adult data, doses usually are scaled based on weight or body surface area. Generally, the body surface area method better reflects age-dependent physiologic differences and is preferred.
PHARMACOKINETICS
Pharmacokinetics describes the movement of drugs throughout the body. Components include absorption of the drug from its site of administration, distribution to tissues and site of effect, metabolism (biotransformation), and elimination. Pharmacokinetic parameters vary with age and should be taken into consideration in selecting drugs to use, routes of administration, and dosing regimens.
Absorption
The rate of drug absorption depends on the dosage form of the drug, the physical–chemical characteristics of the drug, the route of administration, and the individual patient’s physiology. In Figure 7.2, although the total amount of drug absorbed is equivalent based on the area under the curve, the concentration versus time profiles are very different due to differences in the rate of absorption. The time to and magnitude of peak drug concentration and the concentrations achieved at equivalent times differ, which may affect the onset and duration of drug effect. Either profile shown could be desirable, depending on the clinical situation. For example, rapid absorption with higher
peak levels may be desirable for many antimicrobials, anesthetic agents, and analgesics. Slower absorption, with lower peak concentrations but more sustained effects, may be desirable for drugs used to treat chronic conditions such as asthma or hypertension.
peak levels may be desirable for many antimicrobials, anesthetic agents, and analgesics. Slower absorption, with lower peak concentrations but more sustained effects, may be desirable for drugs used to treat chronic conditions such as asthma or hypertension.
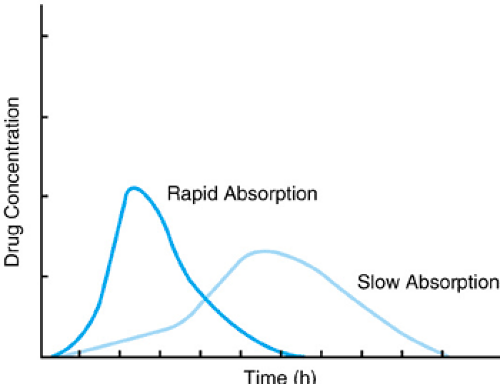 FIGURE 7.2. Differences in rate of drug absorption affect both the magnitude and time of peak drug concentration. |
Drug concentration versus time profiles may differ for the same drug when given by different routes. Subcutaneous or intramuscular administration of morphine may have an onset of action 15 to 20 times slower than a comparable dose given intravenously. A rapidly administered intravenous dose of morphine may produce such high drug concentration with rapid distribution into the central nervous system that respiratory depression may develop.
Developmental changes in several physiologic parameters important for drug absorption occur as the child grows, including stomach and intestinal pH, gastric emptying time, pancreatic function, intestinal flora, and blood flow to the gut. For several drugs, diminished and erratic absorption has been demonstrated in neonates and infants after oral or intramuscular administration. Comorbidities also can affect drug absorption. For example, hypotension and shock lead to decreased blood flow to both the gut and skeletal muscle, making absorption after oral or intramuscular routes unpredictable.
Some drugs display low oral bioavailability because they undergo high first-pass extraction by the liver. This means that a very high percentage of the drug dose is removed from circulation during the initial passage through the liver. The dosage differences for propranolol document the significance of the first-pass effect: The oral dose is 0.1 mg/Kg versus an intravenous dose of 0.01 mg/Kg.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree