Persistent Pulmonary Hypertension
Gross Ian
PATHOGENESIS
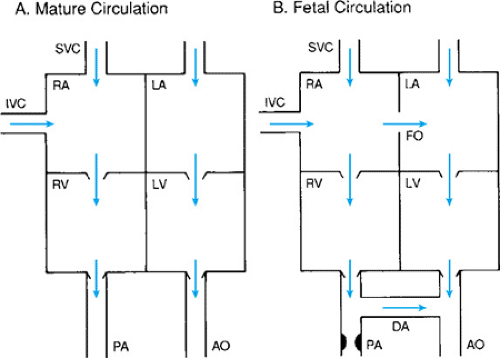
During fetal life the tone in the pulmonary arterial system is increased and the pressure on the right side of the heart is greater than that on the left. Consequently, blood is shunted from the right side of the heart to the left through the foramen ovale and the ductus arteriosus (Fig. 48.1). The blood that enters the right atrium via the superior vena cava tends to flow into the right ventricle, whereas blood that enters the right atrium from the inferior vena cava tends to be shunted across the foramen ovale to the left atrium. Much of the flow from the right ventricle is then shunted to the aorta through the ductus arteriosus. After birth and the onset of breathing, pressure in the pulmonary circulation decreases and functional closure of the foramen ovale occurs, followed by anatomic closure of the ductus arteriosus. In some infants, however, pulmonary hypertension develops
again after birth. This results in reestablishment of right-to-left shunting through the foramen ovale or ductus arteriosus; for this reason, this condition is sometimes referred to as persistent fetal circulation.
again after birth. This results in reestablishment of right-to-left shunting through the foramen ovale or ductus arteriosus; for this reason, this condition is sometimes referred to as persistent fetal circulation.
The pulmonary arterioles are sensitive to hypoxia and acidosis and respond to these stimuli by vasospasm. In infants with lung disease or other causes of hypoxia and acidosis, pulmonary hypertension may develop as a consequence of the hypoxia produced by the underlying disease. This secondary type of pulmonary hypertension usually reverses when the underlying problem has been resolved.
In another group of infants, a more persistent form of pulmonary hypertension develops. They are usually full-term or postmature infants with meconium aspiration or with no lung disease, and a history of asphyxia may be present. The underlying lesion in persistent pulmonary hypertension (PPH) is believed to be abnormal muscularization of the small arterioles supplying the distal airways. At birth, the walls of the arterioles supplying the terminal and respiratory bronchioles are usually only partially muscularized. Infants who die of PPH have been shown at autopsy to have extension of this muscularization into the arterioles that supply the alveolar ducts. Resistance to blood flow occurs as the walls of these arterioles are thickened and the vessel lumen is narrow. Asphyxia or other insults occurring after birth may aggravate the situation by triggering spasm of these thickened arterioles, with resulting severe pulmonary hypertension.
It has been suggested that the abnormal muscularization of the pulmonary vasculature is caused by intrauterine hypoxia during late fetal life. In utero hypoxia also could account for the association of PPH with meconium aspiration and postmaturity. Infants who are stressed in utero, such as postmature infants, are less able to tolerate labor and have a greater tendency to experience intrapartum asphyxia and to pass meconium.
In addition to asphyxia and meconium aspiration, PPH is associated with pulmonary hypoplasia (e.g., diaphragmatic hernia), sepsis, and pneumonia. In diaphragmatic hernia, there is not only pulmonary vasospasm, but also a smaller pulmonary vascular bed in the hypoplastic lung. The hypoxia in these infants may be particularly severe and resistant to therapy. The pulmonary vasospasm in infected infants is thought to be due to vasoactive bacterial toxins.
DIAGNOSIS
The diagnosis of PPH is suggested by the triad of cyanosis, absence of heart disease, and clear lung fields or meconium aspiration. The usual presentation is that of a full-term or postmature infant who may have a history of asphyxia or meconium aspiration. The baby may appear to be well initially, but within the first 24 hours after birth there is progressive cyanosis and tachypnea. A chest radiograph taken at this stage will reveal clear lungs, or scattered infiltrates in the case of meconium aspiration. The lungs may have decreased vascular markings, consistent with diminished pulmonary blood flow. The cyanosis, if untreated, will continue to progress until, eventually, it becomes profound. Shock with decreased peripheral perfusion and hypotension may also develop.
The combination of severe cyanosis with clear lung fields or small infiltrates should raise the suspicion of PPH; the clues are cyanosis that is disproportionate to the radiographic signs of lung disease and a labile (as opposed to a relatively fixed) PaO2 or oxygen saturation. The PaO2 is labile as the pulmonary arterioles are “twitchy,” whereas infants with fixed anatomic heart disease tend to have less variability in the PaO2. However, the infant should not be treated for pulmonary hypertension until heart disease has been excluded, preferably by an echocardiogram. Infants with total anamolous pulmonary venous return with obstruction or other causes of cyanotic heart disease may initially have similar clinical features. The echocardiogram will exclude anatomic heart disease and confirm the diagnosis of pulmonary hypertension and right-to-left shunting at the level of the foramen ovale or ductus arteriosus. It may reveal tricuspid regurgitation resulting from the high right-sided pressure. Echocardiography is also useful for assessing right ventricular filling and myocardial contractility, and can be used as a guide for determining intravenous fluid and pressor requirements.
Comparison of right radial (preductal) PaO2 to umbilical artery (postductal) PaO2 is useful for assessing shunting across the ductus arteriosus. The umbilical PaO2 will be lower in the presence of right-to-left ductal shunting. The absence of such a difference, however, does not rule out PPH and right-to-left shunting, because the shunting may occur at the level of the foramen ovale only. Comparison of upper limb (preductal) to lower limb (postductal) oxygen saturation yields similar information.
THERAPY
Infants with PPH present perhaps the most difficult medical management problem in the newborn intensive care unit, and their care draws on all the resources available to modern neonatology. They should be managed by physicians experienced with this problem and in an environment where the appropriate support is available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree