Pediatric Human Immunodeficiency Virus Infection
Edina H. Moylett
William T. Shearer
As we journey into the twenty-first century, human immuno-deficiency virus (HIV) remains a rapidly expanding modern-day plague. According to the World Health Organization (WHO), at least 42 million people are infected with HIV worldwide, 3.2 million of whom are children younger than 15 years old; 14,000 new infections occur daily, 2,000 of which are in children younger than 15 years of age. Best current projections suggest that an additional 45 million people will become infected with HIV by the year 2010, unless the world succeeds in mounting a drastically expanded global prevention effort.
For those living in most of the developed world, the future for HIV/AIDS (acquired immune deficiency syndrome) is not so bleak. The epidemic of HIV infection among children has changed substantially during recent years, with declining numbers of newly HIV-infected infants coincident with the widespread and effective implementation of recommended antiretroviral therapy to reduce perinatal transmission. In addition, the availability and implementation of highly active antiretroviral therapy (HAART) for use during pregnancy, as well as initial therapy in infected infants and children, have had the effect of further reducing the incidence of transmission and greatly improving the quality of life for HIV-infected individuals. Improved use of prophylaxis for opportunistic infections (OIs) has had an additive effect and further increased the numbers of HIV-infected children surviving into teenage years and beyond.
Despite these advances in the care of individuals with HIV, in excess of 10,000 children are living with HIV infection in the United States, with an estimated annual death rate of at least 100. Despite best efforts at preventing in utero transmission, newly infected infants continue to contribute to the numbers of children who require care for HIV. Adolescents are an emerging at-risk group: 20,000 new cases of HIV infection were reported among individuals 13 to 25 years old in the United States in 2003. With the significant immigrant population in U.S. cities, coupled with these statistics, anyone practicing pediatric medicine today must be familiar with key elements of pediatric HIV infection. Therefore, the purpose of this chapter is to review the fundamental aspects of this largely preventable infectious disease.
EPIDEMIOLOGY
The first cases of pediatric HIV infection were reported in November 1982, approximately 1 year after the first cases in adult patients were reported. In the early 1990s, at the peak of the AIDS epidemic, as many as 30% of pregnant women in the United States transmitted HIV to their newborn infants. This mode of acquisition of HIV accounted for close to 90% of the cases of pediatric HIV infection; the remaining cases were ascribed to the receipt of blood or blood products, sexual abuse, and rarely accidental exposure (e.g., contaminated-needle stick injury). Currently, in the United States and the rest of the developed world, the figures for maternal–fetal transmission have dropped significantly, to less than 2%, thanks primarily to the implementation of potent intrapartum chemoprophylaxis and advances in obstetric care. However, as of the end of 2001, the Centers for Disease Control and Prevention (CDC) National Center for HIV/STD and Tuberculosis Prevention, Division of HIV/AIDS Prevention, reported that almost 86,000 U.S. women of childbearing age (15 to 44 years) are living with HIV/AIDS.
In developing countries, principally sub-Saharan Africa, the story is in stark contrast. Seroprevalence rates of HIV infection among pregnant women are documented to be as high as 40%, and maternofetal transmission rates range from 25% to 40%. Breast-feeding increases the risk of transmission by approximately 15%. As of the end of 2002, figures from The Joint United Nations Programme on HIV/AIDS (UNAIDS) and WHO reported that an estimated .5 million children younger than 15 years of age died as a result of HIV infection, and approximately 2.8 million children were infected with the virus.
In other parts of the world, across all social divides, the HIV epidemic only now is beginning to have a significant impact on the social structure. At the end of 2002, in the Asia-Pacific region, which is home to 60% of the world’s population, 7 million infections already have been recorded, with 1 million new infections reported in the year 2002. According to the United Nations, China can expect to have 10 million cases by 2010, and in India the number of carriers is expected to skyrocket from 4 million to between 20 and 25 million by 2010. Without significant education and assistance from international organizations, the numbers of children born with HIV infection, as well as orphaned by this modern-day plague, will continue on an upward trajectory.
After a plethora of reports in the early to mid-1980s of the development of acquired immunodeficiency syndrome (AIDS) in patients who had received blood products (including clotting factors, blood, and blood products), these blood products were recognized as being sources of the transmission of HIV infection. Since the implementation of universal screening of blood and blood products for antibodies against HIV in 1985, the incidence of acquired HIV infection after receiving blood products has been decreasing from accounting for as many as 20% of pediatric cases in the 1980s to less than 1% today. Additionally, the application of recombinant technology to the production of human blood factors (e.g., factor VIII) has eliminated transmissible disease risk.
PATHOGENESIS
HIV-1, a cytopathic RNA virus in the Retroviridae family, consists of a virion measuring 100 to 150 nm in diameter housing an electron-dense cylindrical core surrounded by a lipid envelope (Fig. 139.1). The envelope, acquired as the virus buds from the host cell, consists of a lipid bilayer containing the
surface (gp120) and transmembrane (gp41) major envelope proteins, in addition to selected host cell-membrane proteins. The viral core, composed of capsid (p24), matrix (p17), and nucleocapsid (p7) structural proteins, contains two copies of single-stranded viral genomic RNA and several virally encoded enzymes (reverse transcriptase, protease, and integrase).
surface (gp120) and transmembrane (gp41) major envelope proteins, in addition to selected host cell-membrane proteins. The viral core, composed of capsid (p24), matrix (p17), and nucleocapsid (p7) structural proteins, contains two copies of single-stranded viral genomic RNA and several virally encoded enzymes (reverse transcriptase, protease, and integrase).
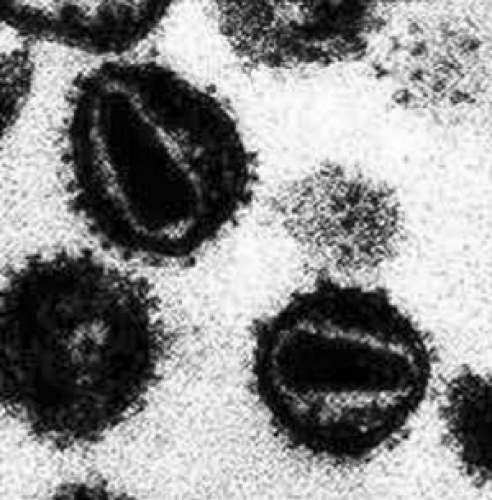 FIGURE 139.1. Human immunodeficiency virus. High-power electron microscopy view of human immunodeficiency virus particles. Note visible central core and envelope glycoproteins (gp120). |
HIV-1 is characterized by its ability to replicate rapidly and mutate within its host. Although an individual may be infected with a predominant strain, as replication ensues, numerous viral variants, also known as quasispecies, are generated.
One of the major milestones in HIV research has been the discovery that chemokine receptors, primarily CCR5 and CXCR4, serve as co-receptors (along with the T-cell recognition molecule CD4+) and allow HIV infection to occur. Additional information about this process is presented in Box 139.1.
Consequent to the discovery of HIV-1 co-receptors was the discovery that polymorphisms of certain chemokines and chemokine receptors may modify the transmission of HIV-1 and progression of disease. The 32-bp deletion in the coding region of the CCR5 gene impedes cellular expression of the HIV-1 co-receptor and provides a strong, but not absolute, resistance to M-tropic viral infection in adults. However, unlike in adults, CCR5 delta-32 homozygosity in children has not been associated thus far with reduced infectivity (vertical transmission); additionally, the heterozygous genotype in children born to HIV-1-seropositive mothers does not confer resistance to infection, but it substantially slows the development of HIV-related disease. Unlike newly infected adult patients, newly infected pediatric patients experience a steep increase in HIV RNA within weeks after birth, followed by a very slow decline in RNA level for several years.
Distinct patterns of disease progression are encountered among perinatally infected infants, those with rapidly progressive disease or “rapid progressors,” and conversely “nonprogressors” or “long-term survivors.” Independent of HAART, some infants are noted to be rapid progressors, whereas others maintain immunologic control of their infection with years (more than 5) of slow decline before the development of AIDS. This phenomenon is not well explained; it is likely that multiple factors, including the aforementioned chemokine polymorphisms, genetically determined functional and structural defects in HIV-1 genes (e.g. nef), as well as a more robust production of HIV antibody and HIV-specific cytotoxic T-lymphocyte (CTL), are at play. Slow progressors constitute approximately 70% to 80% of all HIV-infected children, who have 5 and 6.5-year survival rates of 71% and 68%, respectively.
BOX 139.1 Pathogenesis of HIV
More than 90% of primary HIV infections involve M-tropic (R5, utilizes CCR5) strains, which readily infect macrophages and monocytes in vitro. M-tropic virus typically is non-syncytium inducing. An initial docking step with CD4+ triggers an HIV-envelope conformational change to enable gp120 to bind to CCR5 and initiate viral gp41-mediated virus-cell fusion. The virus replicates efficiently in CD4+/CCR5+-bearing cell types, macrophages, monocytes, and T-cells of lymph nodes, producing some billions of virions per day throughout the typical 10-year and longer course of infection. Most patients infected with subtype (or clade) B HIV strains (the predominant strains in the United States and Europe) experience a mutational transition in their HIV envelope gene, which alters the cell tropism to permit CXCR4 utilization (X4 or T-cell-tropic preference), so that the mutated virus can replicate in CXCR4-bearing cells, including immortalized T-cell lines in vitro. These isolates are encountered more frequently in late-stage disease and are syncytium-inducing. The switch in viral phenotype from R5 to X4 is associated with decline in the number of CD4+ T-cells and rapid progression of disease.
Transmission
The principal mode of transmission of HIV infection among children 15 years of age and younger is maternal-infant transmission, also known as vertical transmission. This mode may occur during pregnancy, immediately prior to birth, or during delivery.
Pregnancy
Current evidence suggests that most maternal-infant transmission of HIV occurs late in pregnancy or during labor and delivery. In a recent Thai study that examined the timing of vertical transmission of HIV among 218 HIV-positive mothers who gave birth to live infants, the overall transmission rate was 22.5%; 5.5% of infections were attributed to in utero transmission as defined by a positive HIV DNA polymerase chain reaction (PCR) at an age of less than 72 hours, and 75.5% of infections were acquired peripartum, as defined by a negative result at birth and a later sample that was HIV-1 positive.
Many risk factors have been identified that increase viral transmission from mother to infant (Table 139.1). Maternal plasma HIV viral load appears to be the best predictor of vertical transmission. Little or no transmission occurs with plasma viral loads of fewer than 1,000 copies/mL regardless of use of zidovudine (ZDV) (also known as azidothymidine [AZT]). Transmission rates between 20% and 60% have been
reported with plasma viral loads ranging from fewer than 100,000 to more than 100,000 copies/mL, respectively. Although the maternal viral load at delivery is very useful for determining the risk of transmission, no level exists above which transmission always occurs nor is there a level below which transmission is never encountered.
reported with plasma viral loads ranging from fewer than 100,000 to more than 100,000 copies/mL, respectively. Although the maternal viral load at delivery is very useful for determining the risk of transmission, no level exists above which transmission always occurs nor is there a level below which transmission is never encountered.
TABLE 139.1. FACTORS ASSOCIATED WITH RISK OF PERINATAL HIV TRANSMISSION | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
The levels of HIV in the maternal genital tract secretions may affect vertical transmission. Antiretroviral therapy (ART) has been shown to decrease HIV RNA levels in cervicovaginal lavage and reduce vertical transmission. Results from the Women–Infant Transmission Study (WITS) in relation to obstetric factors and vertical transmission identified that rupture of membranes 4 hours or more prior to delivery almost doubled the risk (odds ratio, 1.82) for transmission of infection to the infant. Additional factors independently associated with increased transmission were illicit drug use during pregnancy, low antenatal CD4+ lymphocyte count, and a birth weight of less than 2,500 g. Low CD4+ lymphocyte counts and low infant birth weight presumably reflect more advanced maternal disease status and an overall greater risk for transmission of the virus. Additionally, HIV-specific cytotoxic T-lymphocytes are thought to play an important role in the control of HIV replication, thereby slowing disease progression. Low infant birth weight also has been associated with a greater likelihood of in utero transmission occurring.
The role of the placenta in supporting or preventing the vertical transmission of HIV is unclear. In vitro studies indicate that placental trophoblasts actually are infected by HIV-1, mediated by a process that does not utilize those co-receptors normally facilitated for entry of virus (CD4/chemokine receptors). Infection appears to be mediated by the up-regulation of adhesion molecules. In addition, infected T-cell blasts transfer the infection more efficiently than does free virus. Infected placental cells possibly in turn pass the virus to fetal T-lymphocytes in a similar manner, employing integrins and adhesion molecules. These findings would support the increase in the incidence of transmission of HIV in the presence of chorioamnionitis as well as high maternal viral load.
Postpartum and Beyond
Mothers can transmit HIV infection to their offspring in the postnatal period via breast milk. Breast-feeding approximately doubles the risk of vertical transmission, which may occur at any time during the breast-feeding period. In a study from South Africa, which examined vertical transmission in association with breast-feeding, the highest overall rate of vertical transmission occurred in exclusively breast-fed children (39%), with the lowest rate being in infants who never had been breast-fed (24%). Mixed feeding has been shown to further increase the risk for breast milk-related transmission of HIV, possibly because mixed feedings allow both exposure to HIV and increased risk of gastrointestinal infections and the consequent disruption of mucosal integrity. In addition, the risk of HIV transmission from breast-feeding has been estimated to be almost twice as high among women who acquire HIV after birth. HIV viral loads in breast milk correlate with plasma viral loads and reach their peak during and just after seroconversion occurs. More advanced and symptomatic maternal disease, as well as mastitis, have been associated with an increased risk for HIV transmission.
However, the role of breast milk in the transmission of HIV remains somewhat controversial. In developed nations, HIV-infected mothers no longer administer breast milk to their infants, given the increased risk of postpartum transmission. This practice is not feasible in resource-poor countries. Given the unfavorable financial situations, in addition to suboptimal sanitation, in many parts of third-world nations, breast milk often is the sole form of nutrition for newborn infants, especially in their first 3 months of life.
Blood Products
Fortunately, the stringent testing of blood donors has decreased the risk of transfusion-acquired HIV infection significantly.
Blood donations in the United States have been screened for antibody to HIV-1 since March 1985, and for type 2 (HIV-2) since June 1992. Prior to screening for the presence of HIV-1 p24 antigen, an estimated one in 450,000 to 660,000 donations per year were deemed infectious for HIV. With current testing methodology, the risk of acquiring HIV infection through blood products is approximately 1 per 1,000,000 units transfused.
Blood donations in the United States have been screened for antibody to HIV-1 since March 1985, and for type 2 (HIV-2) since June 1992. Prior to screening for the presence of HIV-1 p24 antigen, an estimated one in 450,000 to 660,000 donations per year were deemed infectious for HIV. With current testing methodology, the risk of acquiring HIV infection through blood products is approximately 1 per 1,000,000 units transfused.
Sexual Contact
At present, sexually transmitted HIV infection remains a significant risk for adolescents and young adults who engage in high-risk behaviors. In day-to-day practice, pediatricians must be aware of acute HIV syndrome as a differential diagnosis for an unexplained febrile illness in adolescents and young adults.
DIAGNOSIS
The diagnosis of HIV infection differs in pediatric and adult populations. The transplacental transfer of maternal antibody of the IgG class necessitates performing viral-based confirmatory tests for making early pediatric diagnoses in neonates and infants up to 18 months of age. Thereafter, HIV enzyme-linked immunoabsorbent assay (ELISA) is the standard test, as in adult practice. An early establishment of the diagnosis of neonatal HIV infection is instrumental in the initiation of early and effective antiretroviral therapy that results in improved clinical outcomes.
Currently, of the diagnostic viral-based assays available, HIV DNA PCR is utilized most frequently for infant diagnostic purposes. Qualitative DNA PCR is used to detect cell-associated proviral DNA. Isolation of the virus by peripheral blood lymphocyte co-culture is the gold standard for HIV infection diagnosis; however, high cost and intensive labor requirements limit its use. Culture techniques may be falsely negative in the presence of HAART. Although quantitative plasma RNA PCR assays are available as an alternative for infant diagnostics, care should be exercised when results are in the low quantitative range, given the risk of false-positive results. In the absence of breast-feeding, utilizing viral-based assays, HIV-infected infants can be diagnosed accurately by the time they are 3 to 6 months of age and in some cases as young as 6 weeks old.
The diagnosis of HIV infection after 18 months of age is based primarily on serologic testing. The Western blot (WB) assay (HIV viral proteins of different molecular weights transferred to a polyacrylamide gel) has greater specificity and sensitivity than does the enzyme immunoassay, but it is more labor-intensive. The results of a WB assay are interpreted as follows: negative, absence of all bands; positive, presence of detectable antibodies to at least two of the following three proteins; the core protein (p24) and two envelope proteins (gp41, gp120/160) or three or more bands with one band from each gag, pol, and env; or bands for either p24 or p31 and gp41 or gp120/160; indeterminate, one of the three major bands. An indeterminate result should be repeated; if it persists, a viral-based test should be performed. Detection of a band to p24 antigen commonly is associated with an indeterminate result. Following HIV infection, antibody to p24 antigen typically is the first to develop; results should be positive within 2 to 3 months. Advanced HIV disease or agammaglobulinemia may result in false-negative serology; in such instances, viral-based assays should be used.
The recommended tests used to check for the presence of HIV infection in neonates and infants follow:
Viral-based tests ideally should be performed at birth, between 1 and 2 months of age, and again between 3 and 6 months of age. Acceptable diagnostic viral-based tests include HIV DNA PCR/HIV RNA PCR/HIV lymphocyte co-culture; two of the same tests or any combination of the three tests is acceptable; HIV DNA PCR and HIV co-culture currently are preferred in neonates.
HIV-specific antibody tests (EIA/WB) are performed at birth and again at 6, 12, and 18 months of age until loss of maternal antibodies is documented.
Recommended guidelines for the diagnosis of HIV infection in neonates and infants follow:
HIV infection is confirmed if two consecutive viral-based tests are positive. A positive test should be confirmed with a repeat test on a new specimen as soon as possible.
In the absence of breast-feeding, a perinatally exposed infant is deemed not HIV-infected if viral-based tests are negative up to and including 4 months of age.
Beyond the neonatal period, in the absence of hypogammaglobulinemia or clinical evidence of HIV disease, HIV infection can be reliably excluded if two or more HIV-specific IgG antibody tests performed after the infant reaches 6 months of age, with an interval of at least 1 month between tests, are negative.
After the infant reaches 18 months of age, HIV infection is ruled out with negative HIV IgG serology, absence of clinical disease, and hypogammaglobulinemia and previously negative viral-based tests.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
For the most part, infected newborns are asymptomatic, without evidence of HIV acquisition. Infrequently, infection acquired in utero may manifest in a manner similar to that of other congenitally acquired viral infections, with resultant growth retardation, skin rash, lymphadenopathy, hepatosplenomegaly, and cytopenias. Pediatric HIV disease occurs primarily as a result of maternofetal transmission, and, therefore, the acute viral syndrome encountered in adult patients rarely is appreciated, with the exception of adolescent-age patients practicing high-risk behavior.
Advances in the management of pediatric HIV infection are reflected by the trend in incidence of opportunistic and other infectious complications. During the second decade of the pediatric HIV epidemic, a dramatic decrease has occurred in the frequency of OIs, as reported to the CDC. Advances accountable for this favorable change include the implementation of guidelines to prevent perinatal transmission, development of guidelines and initiation of therapy to prevent OIs, and, finally, an expansion in ART with the formulation of highly active medications suitable for pediatric administration.
Important differences exist between HIV-related clinical entities in adults and those in children:
Prior to the advent of HAART, children had more rapid disease progression.
Pneumocystis jiroveci injection (causing pneumocystis pneumonia PCP) and encephalopathy occur early in rapid progressors. Children have a higher prevalence of bacteremia and lymphocytic interstitial pneumonitis (LIP).
However, Kaposi sarcoma, toxoplasmosis, cryptococcosis, and cytomegalovirus (CMV) infection seldom are encountered.
Additionally, age-related changes in CD4+ T-lymphocyte counts exist, as outlined in Table 139.2.
TABLE 139.2. IMMUNOLOGIC CATEGORIES BASED ON AGE-SPECIFIC CD4+ T-LYMPHOCYTE COUNTS AND PERCENTAGE OF TOTAL LYMPHOCYTES | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


