Chapter 437 Pediatric Heart and Heart-Lung Transplantation
437.1 Pediatric Heart Transplantation
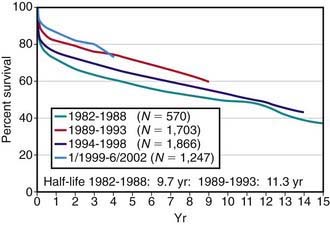
Pediatric heart transplantation is standard therapy for children with end-stage cardiomyopathy and other lesions not amenable to surgical repair. As of 2005, >6,900 heart transplants had been performed on children in the USA, with ≈375 transplants annually. Survival rates among children compare favorably with those of adults. For children transplanted in the 1980s and early 1990s, 1 yr survival has been 75-80%, whereas for those transplanted after 2000, 1 yr survival is now in the range of 90%; during the same time periods, 5 yr survival has improved from 60-65% to 75% (see ![]() Fig. 437-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). A growing number of children are now reaching their 15, 20, and 30 yr post-transplant anniversaries.
Fig. 437-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). A growing number of children are now reaching their 15, 20, and 30 yr post-transplant anniversaries.
Recipient and Donor Selection
Potential heart transplant recipients must be free of serious noncardiac medical problems such as neurologic disease, systemic infection, severe hepatic or renal disease, or severe malnutrition. Many children with ventricular dysfunction may have pulmonary hypertension and even pulmonary vascular disease, which would preclude heart transplantation; pulmonary vascular resistance must be measured at cardiac catheterization, both at rest and in response to vasodilators. Patients with fixed elevated pulmonary vascular resistance are at higher risk for heart transplantation and may be considered candidates for either heterotopic heart transplantation (see later) or heart-lung transplantation (Chapter 437.2). A comprehensive social services evaluation is an important component of the recipient evaluation. Because of the complex post-transplantation medical regimen, the family must have a history of compliance. Detailed informed consent must be obtained.
The decision of when to place a patient on the transplant waiting list is based on a combination of many factors, including extremely poor ventricular function (left ventricular fractional shortening <10%; normal is 28-40%), poor exercise tolerance as determined by cardiopulmonary exercise testing (Chapter 417.5), poor response to medical anticongestive therapy, multiple hospitalizations for heart failure, arrhythmia, progressive deterioration in renal or hepatic function, early stages of pulmonary vascular disease, and poor nutritional status. In patients awaiting transplantation, those with poor left ventricular function (fractional shortening <15%) are usually started on a regimen of anticoagulation to reduce the risk of mural thrombosis and thromboembolism. Patients with cardiogenic shock unresponsive to standard pharmacologic treatment may be candidates for placement of left ventricular (LVAD) or biventricular (BiVAD) assist devices, or for extracorporeal membrane oxygenation (ECMO) support, which can stabilize hemodynamics and serve as a bridge to transplantation (Chapter 436).