Fig. 1
Six hours after a self-inflicted stab wound, this child presented with increasing pain and a “popping” sensation with digital motion. Subcutaneous air is seen at the volar wrist (Courtesy of Kevin Little, MD)
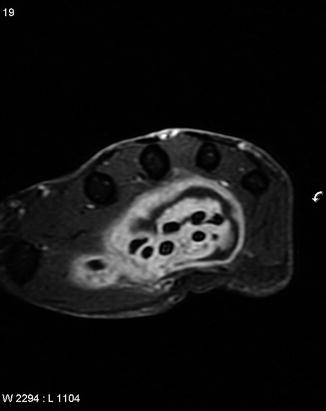
Fig. 2
MRI demonstrates significant fluid collection within the palm, surrounding the flexor tendons. Ultimately, this infection was diagnosed as an atypical mycobacterial infection
Laboratory workup should also be performed for all but the simplest of hand infections. Routine inflammatory laboratory tests including a complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) should be obtained to establish a baseline upon which further treatment can be based. Blood cultures and wound cultures, when possible, also are recommended prior to the initiation of antibiotics.
Once the workup is complete, and a working diagnosis is established, treatment is initiated. Empiric antibiotics can be initiated once cultures are obtained. Table 1 presents options for empiric antibiotic treatment depending on the etiology of the infection.
Table 1
Empiric Treatment for Common Pediatric Hand Infections
Type of infection | Most common organism | Less common organisms | Empiric antibiotic coverage |
|---|---|---|---|
Acute paronychia | Staphylococcus aureus | Dicloxacillin | |
Beta-hemolytic streptococci | Cephalexin | ||
Amoxicillin-clavulanate | |||
Clindamycin | |||
Chronic paronychia | Fungi | Topical corticosteroids and antifungals (usually no role for systemic antimicrobials) | |
Mycobacterial | |||
Bacterial (rare) | |||
Felon | Staphylococcus aureus | Dicloxacillin | |
Oral anaerobes | Cephalexin | ||
Amoxicillin-clavulanate | |||
Clindamycin (PCN allergic) | |||
Herpetic whitlow | Herpes simplex virus 1 or 2 | Acyclovir (preferred) | |
Valacyclovir or famciclovir (alternatives) | |||
Flexor tenosynovitis | Staphylococcus aureus | Pasteurella multocida | Community acquired |
Gram-negative rods | Kingella kingae | Vancomycin plus cefotaxime | |
Enterobacter spp. | Vancomycin plus ciprofloxacin (older adolescents) | ||
Sporothrix schenckii | Postoperative | ||
Alternaria spp. | Vancomycin plus cefepime and metronidazole | ||
Mycobacteria | Vancomycin plus piperacillin-tazobactam | ||
Deep space infections | Staphylococcus aureus | Community acquired | |
Anaerobes | Vancomycin plus ampicillin-sulbactam | ||
Gram-negative rods | Vancomycin plus moxifloxacin (older adolescents) | ||
Vancomycin plus cefotaxime and metronidazole | |||
Postoperative | |||
Vancomycin plus cefepime | |||
Vancomycin plus piperacillin-tazobactam | |||
Human bites | Staphylococcus aureus | Actinomyces | Amoxicillin-clavulanate (superficial) |
Eikenella corrodens | Treponema pallidum (syphilis) | Ampicillin-sulbactam (deep) | |
Streptococcus spp. | Mycobacterium tuberculosis | Clindamycin plus trimethoprim-sulfamethoxazole (PCN allergic) | |
Anaerobes | Hepatitis B virus | ||
Bacteroides melaninogenicus | |||
Animal bites | Gram-positive cocci | Bacteroides spp. | Amoxicillin-clavulanate (superficial) |
Anaerobes | Viridans streptococci | Ampicillin-sulbactam (deep) | |
Pasteurella multocida | Clindamycin plus trimethoprim-sulfamethoxazole (PCN allergic) | ||
Septic arthritis | Staphylococcus aureus | Community acquired | |
Streptococcus spp. | Vancomycin plus cefotaxime or ceftriaxone (especially if gonococcal suspected) | ||
Neisseria gonorrhoeae | Vancomycin plus ciprofloxacin (older adolescents, non-gonococcal) | ||
Postoperative | |||
Vancomycin plus cefepime | |||
Vancomycin plus piperacillin-tazobactam | |||
Osteomyelitis | Staphylococcus aureus | Streptococcus pneumoniae | Community acquired |
Streptococcus spp. | Bartonella henselae | Vancomycin plus cefotaxime or ceftriaxone | |
Gram-negative rods | Prevotella spp. | Vancomycin plus ciprofloxacin (older adolescents) | |
Porphyromonas spp. | Postoperative | ||
Vancomycin plus cefepime and metronidazole | |||
Vancomycin plus piperacillin-tazobactam |
Cases that present early, and without any systemic toxicity, can be treated nonoperatively (Table 2). Appropriate treatment in this early stage includes soft tissue rest via splinting, usually in a forearm-based plaster resting splint. Elevation with the use of a sling or foam pillow is also important, along with empiric antibiotic administration. However, any systemic toxicity or the presence of a developing infection for over 48 h should prompt a decision for surgical intervention.
Table 2
Indications and Contraindications for Nonoperative Treatment of Pediatric Hand Infections
Nonoperative management | |
|---|---|
Indications | Contraindications |
Presentation within 24 h of onset of symptoms | Obvious underlying fluctuance |
Mild appearance without systemic signs | Systemic toxicity |
No focal fluid collection is present | Immunocompromised host |
Classification of Hand Infections
Hand infections can be classified into three broad categories: superficial spreading infections, closed space infections, and other infections. Superficial spreading infections include conditions such as cellulitis and necrotizing fasciitis. It is important to acknowledge that cellulitis can coexist with a deeper infection, and this possibility should always be at the forefront of the treating surgeon’s mind. If a cellulitis fails to resolve or worsens within 24 h of initiation of appropriate antibiotic treatment, other deeper sources of infection should be sought. Careful attention should also always be paid to the spread of superficial infections and the rate. Rapid spread of skin infections should prompt immediate further evaluation and a trip to the operating room, if necessary, to assess for a surgical emergency such as necrotizing fasciitis.
Closed space infections are characterized by a contained collection of purulence. The treatment for any of these is surgical drainage; antibiotics alone are insufficient treatment, since the antibiotics will not penetrate or cause resolution of a fluid collection.
Other infections will be addressed individually below and include atypical infections (viral, fungal, and mycobacterial), bite wounds, and osteomyelitis.
Outcome Tools
No specific outcome tools are available for the assessment of hand infections. Appropriate evaluation of the outcomes of treatment for hand infections would include the Disabilities of the Arm, Shoulder and Hand (DASH) or QuickDASH or other anatomically specific outcome tools.
Occupational Therapy Recommendations
Early management of hand infections consists of immobilization in a position of comfort, which a hand therapist can assist with as necessary, though plaster or fiberglass splints are more commonly used. Once there is evidence of improvement, gentle range of motion and edema control should be initiated with the assistance of a hand therapist if the patient is at an age where they can follow instructions. If the edema associated with the infection is allowed to persist, and the extremity is rested for too long, stiffness becomes a much more significant problem.
Surgical Treatment
Surgical management of hand infections is simple in principle. If there is underlying purulence, it must be evacuated expeditiously and thoroughly. The method of anesthesia is dependent upon the type of infection being treated. However, when deep infections are present, general anesthesia is preferred for the patient’s comfort. A standard instrument set for hand surgery is typically adequate to perform an irrigation and debridement procedure (Table 3). In general, these cases are performed under tourniquet control, but the limb should NOT be exsanguinated with an elastic bandage (Esmarch), as this might actually “push” the bacteria up the arm and cause more systemic spread of the bacteria. Gravity exsanguination is preferred.
Table 3
Preoperative planning
OR table: supine on a standard OR bed with attached hand table |
Position/positioning aids: For digital infections that will be approached from volarly, a lead or aluminum hand or other hand positioning device is useful |
Fluoroscopy: Fluoroscopy is not usually required for an irrigation and debridement procedure, unless bony debridement is anticipated, such as in cases of osteomyelitis |
Equipment |
Low-pressure irrigation tubing (e.g., cystoscopy tubing) versus bulb irrigation |
Standard hand surgery tray, equipped with skin hooks, traumatic and atraumatic forceps, dissecting scissors, hemostats |
Pediatric feeding tubes if closed catheter irrigation is anticipated |
Penrose or other appropriate small drains |
Tourniquet: An upper arm tourniquet is almost invariably used, though the arm should be exsanguinated with gravity, and not with an elastic (Esmarch) bandage |
Incisions should be planned carefully and will be more fully described below in conjunction with the different types of infection. However, special care should be taken to avoid incision lines that cross flexion creases at a right angle, since such incisions are prone to creating scar contractures. Bruner incisions should also be avoided in the digits, if possible, as there is a higher risk for tip necrosis of flaps with narrow bases in the setting of a hand compromised by infection.
Once a pocket of purulence is opened, cultures should be obtained. Since the etiologic organisms can be varied, a good rule of thumb is to send at least aerobic and anaerobic cultures, fungal cultures, and acid-fast cultures. It is important to recognize that most laboratories will require tissue for acid-fast cultures. The uncovered pocket of purulence should then be copiously irrigated. It is critical to thoroughly and systematically explore all edges of the infected area and to probe fairly aggressively in order to convince oneself that no further pockets of purulence remain. Once a thorough irrigation and debridement of any necrotic tissue has been performed, a loose closure over a drain or open packing should be performed. The limb is then placed in a splint for soft tissue rest for 24–48 h.
Microbiology
Most epidemiologic studies agree that Gram-positive organisms including Staphylococcus aureus and beta-hemolytic streptococci are the most common organisms responsible for infections in the adult hand, accounting for 40–80 % of cases (Fowler and Ilyas 2013; Tosti and Ilyas 2010; Houshian et al. 2006; McDonald et al. 2011). However, what is less clear is the extent to which this data translates to children. Harness and Blazar (2005) reviewed a series of 31 pediatric patients with hand infections and found that Staphylococcus aureus was the primary organism in 37 % and that the rate of mixed and anaerobic infections was proportionally higher in pediatric patients than in adults. Based on their data, the authors recommended the use of more broad-spectrum coverage as empiric treatment for pediatric hand infections.
Also of particular concern is the rising prevalence of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) . This trend is well documented in the adult population, in which community-acquired MRSA has been reported to be present in between 30 % and 80 % of cases (Tosti and Ilyas 2010; Fowler and Ilyas 2013; O’Malley et al. 2009). More recently, Chung et al. (2012) reported on a cohort of 415 patients younger than 15 years old with hand infections, with the rate of MRSA-positive cultures being 30 %. Because of this growing body of data, careful attention should be paid to the local prevalence of MRSA. If the local rate is >10 %, then empiric treatment for all hand infections should avoid 1st-generation cephalosporins. For milder cases, treatment with trimethoprim-sulfamethoxazole orally is recommended. For more involved cases, admission to the hospital and intravenous vancomycin are indicated (Chung et al. 2012).
Though the most common organisms are Staphylococcus spp. and Streptococcus spp., the list of other organisms that can be implicated in hand infections is long and diverse. Thus, care and attention should be given to other risk factors that might play a role (Table 1). Particularly in milder or more indolent cases, consideration should be given to the possibility of an atypical infection from a fungal or mycobacterial organism. While such atypical infections are rare, the treatment required for eradication of the infection is markedly different and must be tailored accordingly. Specific atypical organisms will be discussed below.
Finger Infections
Acute Paronychia
An infection of the skin folds around the nail plate, otherwise known as a paronychia , is one of the most common infections in the pediatric hand. Both acute and chronic forms of paronychia exist. While the acute infection can often be associated with a “hangnail,” nail biting, splinters, or other minor trauma, the chronic infections are often the result of a persistently moist environment. In children, this is usually the result of persistent thumb or digital sucking (Harness and Blazar 2005; Stone and Mullins 1968; Rayan and Turner 1989). In addition, acute infections may present superimposed on a chronic infection.
It is important to determine historically whether or not a chronic infection is present, as the microbiology associated with a chronic paronychia is significantly different from that of an acute paronychia. As such, the treatment is also different. In the acute form, Staphylococcus aureus is the usual causative organism. However, because the etiology of paronychia in children can be digital sucking, a mixed organism infection or atypical organisms may be present. For chronic infections, atypical organisms such as fungi are usually implicated.
The typical presentation of a paronychia is localized pain, erythema, and swelling along the paronychial fold. While these infections are most commonly found on only one side of the nail plate, they can sometimes progress around the proximal (eponychial) fold to include the other side in a “runaround” abscess (Green et al. 2011).
Operative Technique
Treatment of acute paronychia in children is similar to the treatment in adults (Table 4). If it is caught in the earliest stages and only localized erythema is present without obvious fluctuance or purulence, then treatment with oral antibiotics, elevation, and warm compresses is appropriate with close observation. However, if this treatment has not been effective after 24 h or if there is any evidence of purulence, then a more aggressive approach is warranted. If the patient is able to tolerate a local digital block, then this method is preferred. However, in young children the use of conscious sedation can relieve the anxiety of both the child and the parents.
Table 4
Incision and drainage of acute paronychia
Surgical steps |
|---|
A blunt instrument such as a Freer elevator can be used to lift the affected paronychial fold |
Alternatively, a longitudinal incision is made along the fold |
If obvious purulent material is present under the nail plate, then a Freer is used to gently slide under the nail plate, and either a portion or the entire nail plate is removed |
If the infection extends proximal to the eponychial fold, then one or two longitudinal incisions are extended in line with the paronychia, and the eponychial fold is elevated to expose the underlying germinal matrix |
Once the infected area is exposed, it is thoroughly irrigated, with gentle debridement using the blunt Freer |
A small strip of gauze or the native nail plate can then be gently placed back under the eponychial fold to preserve that space |
Once anesthesia is achieved, surgical drainage is performed. The method of drainage depends on the location of the purulence, with the bottom line of achieving complete surgical drainage of any underlying purulence. In simple paronychia localized to the paronychial fold, a Freer elevator can be used to gently develop the plane between the nail plate and the paronychia, or alternatively, a longitudinal incision in line with the paronychial fold is adequate to relieve tension. Once the infection progresses, purulence can track underneath the nail plate. If this is seen, partial or complete nail plate removal is necessary. Depending on how proximally the infection tracks, the eponychial fold can also be lifted through the use of longitudinal incisions in line with the paronychia (Fig. 3).


Fig. 3
A longitudinal incision, in line with the nail plate, can be used to decompress the paronychia as seen on the index finger. If the infection tracks proximally to involve the eponychial fold, then incisions can be extended parallel to the paronychial folds to permit lifting the eponychial fold away from the nail plate as seen on the long finger
Chronic Paronychia
In the case of a chronic paronychia, the offending organism must be identified. The clinical course of a chronic paronychia is often indolent, with periodic flares of symptoms that do not rapidly progress as in the acute infection. Treatment is often difficult because of the mixed microbiology responsible for these infections, which can include bacterial, mycobacterial, and fungal components.
The mainstay of treatment for chronic paronychial infections is topical creams that contain a combination of antifungals and steroids (3 % clioquinol in triamcinolone-nystatin mixture). However, in refractory cases, marsupialization is necessary. Marsupialization involves removing a full-thickness, crescent-shaped segment of the skin proximal to the eponychial fold (a “pocket”) that is then allowed to freely drain and heal by secondary intention. This procedure is done with or without the removal of the nail plate depending on the involvement of the nail bed and the nail plate itself (Canales et al. 1989; McDonald et al. 2011; Green 2005).
Felon
A felon is a closed space infection involving the pulp of the fingertip. The anatomy of the fingertip is characterized by the presence of multiple septa, each of which can create walled-off collections of purulence if inoculated (McDonald et al. 2011). With the fingers of the hand as the primary means of connecting with the environment, it is no surprise that these are some of the more common infections seen in the pediatric hand. In adults, these infections are most commonly caused by Staphylococcus aureus. However, the bacterial etiology of felons in the pediatric population is not known.
The source of inoculation can be anything from a plant thorn to a pet bite (in particular cat bites), to penetration of the fingertip with a household object such as a sewing needle. The presentation involves throbbing pain of the affected finger, accompanied by erythema and swelling localized to the fingertip pulp. While sometimes the inoculation wound can be seen, often there is no obvious wound present.
If the inoculated finger is caught early, the infection may be treated effectively with elevation, oral antibiotics, and warm compresses. However, more commonly these infections have already progressed to include an abscess (Canales et al. 1989; McDonald et al. 2011). In these cases, early surgical drainage is essential, since untreated abscesses within the fingertip can quickly lead to more extensive infections. The building pressure of purulence within the pulp of the digit can cause local spread to the underlying bone, leading to osteomyelitis, or can travel more proximally to seed the flexor tendon sheath or the distal interphalangeal joint resulting in a pyogenic flexor tenosynovitis or a septic arthritis, respectively. These infections should be treated early and aggressively to avoid such complications.
Operative Technique
Surgical treatment is performed under local anesthesia with or without conscious sedation or under general anesthesia (Table 5). There is no agreement on the optimal incision for surgical drainage, though the author’s preference is for a high lateral incision placed just below the level of the nail plate on the affected side (Fig. 4). This incision prevents disturbance of the volar fat pad and neurovascular bundle. Though in many cases, the placement of the incision is based on the location of a traumatic wound, when possible, careful consideration of the placement of the incision can avoid significant postoperative morbidity. Thumb and small finger incisions are best made radially, while other digits should be approached from the ulnar side to avoid the primary areas involved in pinch. Once the incision is made, aggressive spreading with a small hemostat or dissecting scissors is necessary to break up any septa and ensure a complete surgical decompression. A small drain should be left in place for a day or two to prevent additional accumulation. Splinting, strict elevation, and appropriate antibiotics are then initiated. When there is clinical evidence of improvement, usually at about 48 h after drainage has been performed, the drain is removed and the wound is allowed to heal by secondary intention. A standard postoperative protocol can be found in Table 7.
Table 5




Incision and drainage of Felon
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


