Endometrial stromal and related tumors
Low-grade endometrial stromal sarcoma
High-grade endometrial stromal sarcoma
Undifferentiated uterine sarcoma
Uterine tumor resembling ovarian sex cord tumor (UTROSCT)
Smooth muscle tumors
Leiomyosarcoma
Epithelioid variant
Myxoid variant
Smooth muscle tumor of uncertain malignant potential
Mixed epithelial and mesenchymal tumors
Adenosarcoma
Carcinosarcoma
Miscellaneous mesenchymal tumors
Rhabdomyosarcoma
Perivascular epithelioid cell tumor (“PEComa”)
Other malignant mesenchymal tumors
A practical classification of uterine sarcomas excluding carcinosarcoma, with incidence [4], is as follows:
Leiomyosarcoma (LMS 60 %) |
Endometrial stromal sarcoma (ESS 30 %) |
Undifferentiated uterine sarcoma (UUS 5%) |
Adenosarcoma and other uterine sarcomas (5 %) |
The 1988 International Federation of Gynecology and Obstetrics (FIGO) criteria for endometrial carcinoma have been used until now for staging uterine sarcomas in spite of the different biologic behavior of both tumor categories. The new FIGO staging system, approved by the FIGO executive board in September 2008, is specifically designed for uterine sarcomas to reflect different biologic behavior [5]. It includes: (1) staging for leiomyosarcoma (LMS) and endometrial stromal sarcoma (ESS), (2) staging for adenosarcomas (AS), and (3) staging for carcinosarcomas, of which the first two are new, while carcinosarcomas will continue to be staged according to the new classification of endometrial carcinoma.
FIGO Staging System for Uterine Sarcomas (2009)
1.
Leiomyosarcomas and endometrial stromal sarcoma
Stage I Tumor limited to uterus |
IA Less than or equal to 5 cm |
IB More than 5 cm |
Stage II Tumor extends beyond the uterus, within the pelvis |
IIA Adnexal involvement |
IIB Tumor extends to extrauterine pelvic tissue |
Stage III Tumor invades abdominal tissues (not just protruding into the abdomen) |
IIIA One site |
IIIB More than one site |
IIIC Metastasis to pelvic and/or para-aortic lymph nodes |
Stage IV |
IVA Tumor invades bladder and/or rectum |
IVB Distant metastasis |
2.
Uterine adenosarcomas
Stage I Tumor limited to uterus |
IA Tumor limited to endometrium/endocervix with no myometrial invasion |
IB Less than or equal to half myometrial invasion |
IC More than half myometrial invasion |
Stage II Tumor extends beyond the uterus, within the pelvis |
IIA Adnexal involvement |
IIB Tumor extends to other pelvic tissues |
Stage III Tumor invades abdominal tissues (not just protruding into the abdomen) |
IIIA One site |
IIIB More than one site |
IIIC Metastasis to pelvic and/or para-aortic lymph nodes |
Stage IV |
IVA Tumor invades bladder and/or rectum |
IVB Distant metastasis |
3.
Carcinosarcomas
Carcinosarcomas should be staged as carcinomas of the endometrium.
Note: Simultaneous tumors of the uterine corpus and ovary/pelvis in association with ovarian/pelvic endometriosis should be classified as independent primary tumors.
The 2009 FIGO staging systems make no mention of undifferentiated uterine sarcoma or pure heterologous sarcomas, such as rhabdomyosarcoma. These tumors are staged in the same way as leiomyosarcoma and endometrial stromal sarcoma. The term “uterus” includes the uterine corpus and uterine cervix, and the 2009 FIGO staging system is used for all uterine sarcomas, irrespective of whether tumors arise in the corpus or cervix.
Leiomyosarcoma
Leiomyosarcomas are malignant neoplasms that demonstrate either histological or immunohistochemical smooth muscle differentiation. Although LMSs represent only about 1 % of all uterine malignancies, they are the most common pure uterine sarcomas accounting for 25–30 % of uterine sarcomas. They occur almost exclusively in adults and in an older age group than leiomyomas, with the median age being 50–55 years. LMSs are aggressive tumors that have a tendency to spread locally, regionally, or by hematogenous dissemination, most commonly to the liver and lung. The most common presenting symptoms are abnormal vaginal bleeding and pelvic pain. Local and regional spread may produce an abdominal or pelvic mass with gastrointestinal or urinary tract symptoms or hemoperitoneum. LMSs are generally thought to arise de novo, but recent molecular genetic evidence suggests that some of these tumors may evolve from preexisting leiomyomas [6].
Gross Features
LMSs are characteristically solitary, intramural masses and are usually not associated with leiomyomas. The gross appearance of LMSs has significant differentiating features from that of leiomyomas in most of the cases. They are large tumors averaging 10 cm in diameter with poorly circumscribed borders that appear to irregularly infiltrate the adjacent myometrium. Leiomyomas, on the other hand, characteristically have a sharp line of demarcation separating the tumor from the myometrium. LMSs that present as a circumscribed mass are not usually recognized grossly as a malignant tumor because of the overlap in appearance between malignant change and the various forms of degeneration. The macroscopic features that are suspicious of malignancy and require thorough sampling of the tumor are: loss of whorled pattern, heterogeneity, irregular, merging, blurred or poorly defined margins, yellow, tan or gray color, softer tumors that lack a rubbery, resilient feel, and absence of a bulging surface. The cut surface may also show irregular areas of hemorrhage and necrosis.
Microscopic Features
LMSs exhibit hypercellularity, severe nuclear atypia, geographic foci of tumor cell necrosis, and a high mitotic rate including atypical mitotic figures, generally exceeding 15 mitotic figures per 10 high-power fields (Fig. 11.1a). Mitosis is counted in the most mitotically active areas in ten successive HPFs using an 40× objective and a standard 10× eyepiece. The poorly differentiated tumors show nuclear pleomorphism, hyperchromasia, prominent nucleoli, and tumor giant cells, features that indicate increasing anaplasia of the tumor (Fig. 11.1b). Well-differentiated LMS on the other hand consist of elongated smooth muscle cells with regular nuclei that differ little from those of a leiomyoma (Fig. 11.1c). Areas of coagulative necrosis and hemorrhage are seen (Fig. 11.1d). The tumors, in most cases, would have invaded the adjacent myometrial tissue at the time of diagnosis and may have perforated the serosal surface of the uterus with involvement of other pelvic organs. Vascular invasion may also be seen.
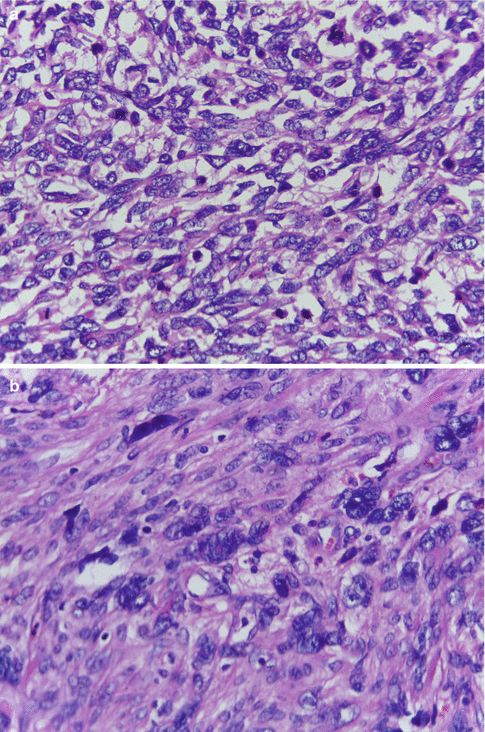
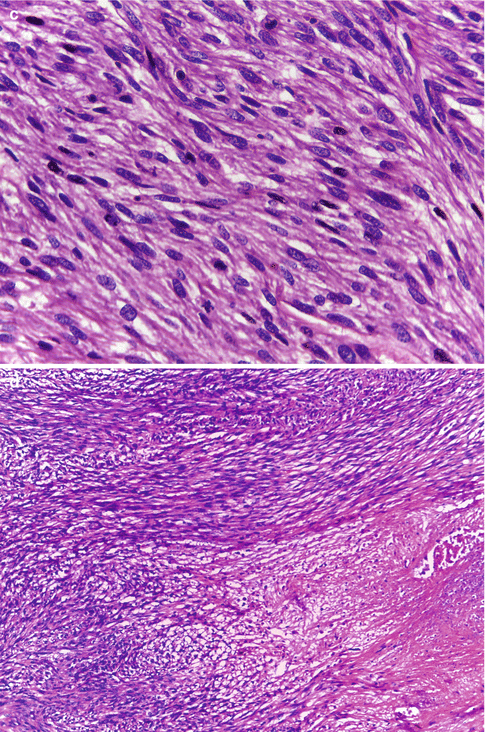
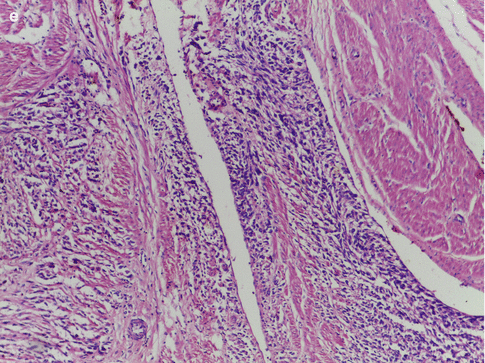



Fig. 11.1
(a) Leiomyosarcoma with hypercellularity, hyperchromatism, nuclear atypia, and high mitotic activity. (b) Leiomyosarcoma with nuclear pleomorphism and tumor giant cells. (c) Leiomyosarcoma with mild nuclear atypia and high mitotic activity. (d) Leiomyosarcoma with foci of coagulative necrosis. (e) Leiomyosarcoma with infiltrative margins
If a smooth muscle tumor is well circumscribed, composed of cells that are uniform in size and shape, has no intravascular component, cytological atypia or necrosis, and with a mitotic count of less than 5 mitotic figures per 10 HPFs, then the tumor is a leiomyoma. On the other hand, when a tumor has infiltrative margins (Fig. 11.1e), intravascular growth, marked cytological atypia, coagulative tumor cell necrosis, a mitotic count greater than 10 mitosis per 10 HPFs and abnormal mitotic figures, then the tumor is an LMS. Most of the smooth muscle tumors belong to the two extremes of the spectrum and are either clearly benign or malignant. It is the tumors that fall in the intermediate category that require careful consideration to reliably categorize them, to predict prognosis, and to decide on further treatment. Numerous studies have been undertaken during the past three decades to define the histological criteria that would help to correctly categorize these tumors and to differentiate them from leiomyomas with atypical histological features like the mitotically active leiomyoma, cellular leiomyoma, atypical leiomyoma, myxoid leiomyoma, epithelioid leiomyoma, hemorrhagic leiomyoma, leiomyoma with hormone-induced changes, and from leiomyomas with unusual growth patterns like disseminated peritoneal leiomyomatosis, benign metastasizing leiomyoma, intravenous leiomyomatosis, and lymphangioleiomyomatosis. Of the many histological features that were assessed, mitotic index, the degree of cytological atypia, and the presence or absence of coagulative tumor cell necrosis have emerged as the most important predictors of behavior [7]. No single histological feature, with the exception of tumor cell necrosis, is diagnostic of malignancy. By employing three variables in the assessment of smooth muscle tumors, this diagnostic strategy moves away from complete dependence on mitotic count [8].
Other features that need to be considered are age of the patient, the size of the tumor and its gross appearance, the tumor’s margins, and presence of vascular invasion.
The epithelioid and myxoid variants of LMS are rare tumors that exhibit malignant biologic behavior despite low mitotic counts and mild nuclear atypia.
LMSs are immunoreactive to smooth muscle markers such as smooth muscle actin, desmin, and h-caldesmon and histone deacetylace. Epithelioid LMS can express epithelial markers such as keratin and epithelial membrane antigen and are often immunoreactive to CD10.
Mutation and over expression of p53 and p16 has been described in a significant minority of LMS and in STUMPs that are at an increased risk of aggressive behavior but not in leiomyomas [9]. However other studies have shown that there is significant overlap in the Ki-67, p16, and p53 expression patterns in the atypical leiomyoma to LMS spectrum precluding its routine use for diagnostic purpose.
Smooth Muscle Tumor of Uncertain Malignant Potential
Uterine smooth muscle tumors that show some worrisome histological features like necrosis, nuclear atypia, or mitosis, but do not meet all the generally applied diagnostic criteria for LMS, fall into the category of STUMP. Tumors are categorized as STUMP when: (a) The tumor has a moderately high mitotic count and some nuclear atypia, but it is not clear whether the tumor belongs to the usual, myxoid, or epithelioid type, making it difficult to apply the relevant guidelines to determine malignancy. (b) The tumor exhibits diffuse significant atypia, but the mitotic index is borderline between atypical and malignant categories. (c) The tumor is hypercellular, lacks tumor cell necrosis, has an MI >10, and exhibits borderline nuclear atypia. (d) The tumor has focal significant atypia, MI > 10, and no tumor cell necrosis. (e) Tumor cell necrosis is present in a hypercellular neoplasm, but there is no significant atypia and the mitotic index is <10. (f) The tumor has diffuse significant atypia or a MI >10, and has necrosis of ambiguous type [10]. When the differential diagnosis is between STUMP and LMS, the diagnosis of STUMP should be favored when the tumor is small (<3 cm), since malignant behavior in a primary smooth muscle tumor <3 cm is yet to be reported. A STUMP diagnosis in a myomectomy specimen allows for flexibility in management that would not be available to patients with a diagnosis of LMS. However, the term should be used sparingly and every effort should be made to classify a smooth muscle tumor into a specific category.
Diagnostic Criteria for Smooth Muscle Tumors [11]
Spindle Cell Smooth Muscle Tumors with Significant Nuclear Atypia
Diffuse or multifocal moderate – severe nuclear atypia + no tumor cell necrosis + > 10 mitotic figures/10 high-power fields = Leiomyosarcoma
Diffuse, multifocal, or focal moderate – severe nuclear atypia + tumor cell necrosis + > 10 mitotic figures/10 high-power fields = Leiomyosarcoma
Diffuse, multifocal, or focal moderate – severe nuclear atypia + no tumor cell necrosis + < 7 mitotic figures/10 high-power fields = Atypical leiomyoma
Diffuse or multifocal moderate – severe nuclear atypia + no tumor cell necrosis + > 7 but < 10 mitotic figures/10 high-power fields = STUMP
Spindle Cell Smooth Muscle Tumors without Significant Nuclear Atypia
No or minimal nuclear atypia + tumor cell necrosis + > 10 mitotic figures/10 high-power fields = Leiomyosarcoma
No or minimal nuclear atypia + tumor cell necrosis + < 10 mitotic figures/10 high-power fields = STUMP
No or minimal nuclear atypia + no tumor cell necrosis + > 5 but < 15 mitotic figures/10 high-power fields = Mitotically active leiomyoma
No or minimal nuclear atypia + no tumor cell necrosis + > 15 mitotic figures/10 high-power fields = Mitotically active leiomyoma (uncertain behavior)
Myxoid Smooth Muscle Tumors
<2 mitotic figures/10 high-power + no tumor cell necrosis + no to minimal nuclear atypia + no infiltration of myometrium = Myxoid leiomyoma
>2 mitotic figures/10 HPFs or tumor cell necrosis or moderate to severe nuclear atypia or infiltration of the myometrium = Myxoid leiomyosarcoma
Epithelioid Smooth Muscle Tumors
<3 mitotic figures/10 high-power fields + no tumor cell necrosis + no to minimal nuclear atypia + no vascular invasion + well circumscribed margin = Consider epithelioid leiomyoma
>3 mitotic figures/10 high-power fields or tumor cell necrosis or moderate to severe nuclear atypia or vascular invasion or infiltrative margin = Epithelioid leiomyosarcoma
Endometrial Stromal Sarcoma
Endometrial stromal tumors are rare tumors derived from the endometrial stromal cells and are composed of cells resembling proliferative phase endometrial stroma. The tumors are currently classified by the WHO as endometrial stromal nodule, low-grade endometrial stromal sarcoma, high-grade endometrial stromal sarcoma, and undifferentiated uterine sarcoma. The tumors account for approximately 0.2 % of all malignant uterine tumors and 10–15 % of uterine sarcomas.
Malignant endometrial stromal tumors were subclassified for many years into low-grade and high-grade ESSs according to their degree of mitotic activity. Tumors with <10 mitotic figures per 10 high-power fields were low grade and tumors with >10 mitotic figures per 10 high-power fields were high grade. However, subsequent studies of uterine sarcomas with recognizable endometrial stromal differentiation failed to confirm the prognostic relevance of this separation based upon mitotic activity, and found surgical stage to be the most powerful predictor of clinical outcome [12]. This resulted in the elimination of the high-grade ESS category based upon a high mitotic rate, and the low-grade ESS were referred to simply as ESS. However, many investigators favored the acceptance of a category of ESS with more than the usual degree of nuclear atypia seen in low-grade ESS and with significant mitotic activity yet retaining some evidence of endometrial stromal differentiation. More recently, some tumors previously considered to be undifferentiated uterine sarcomas have been shown to be of endometrial stromal derivation (often associated with a component of low-grade endometrial stromal neoplasm) and are designated high-grade endometrial stromal sarcomas. The 2014 WHO classification of tumours of the uterine corpus includes the category of high-grade endometrial stromal sarcoma in the endometrial stromal and related tumors group [3].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


