DANIELA E. MATEI  HELEN MICHAEL
HELEN MICHAEL  DAVID M. GERSHENSON
DAVID M. GERSHENSON
INTRODUCTION
Significant improvement in the management of ovarian germ cell tumors (OGCT) has been achieved during the past 3 decades. The development of more effective chemotherapy regimens is the leading cause for improved outcome in this rare group of patients. Other advancements in the field include the development of a more precise surgical staging system, improved radiographic imaging, more sophisticated pathology techniques, as well as improved supportive care and symptom control that has enabled safe delivery of treatment. A substantial majority of patients with OGCT are long-term survivors and suffer minimal morbidity from treatment. Fertility-sparing surgical procedures enable young women with OGCT to preserve their reproductive potential. These positive results in clinical outcome reflect a collaborative effort between different specialties (surgery, medical oncology, pathology, and radiology).
PATHOLOGY
The current World Health Organization (WHO) classification of OGCTs includes dysgerminoma, yolk sac tumor, embryonal carcinoma, polyembryoma, nongestational choriocarcinoma, mixed germ cell tumors, and teratomas (immature, mature, and monodermal types) (1). Primitive germ cell tumors account for 2% to 3% of all ovarian cancers and occur usually in young women. The peak age incidence for development of these tumors is the early twenties. The pathology of these neoplasms is discussed here in the same order as the listing in the current WHO classification.
Dysgerminoma
Dysgerminoma represents the most common ovarian malignant germ cell tumor (2). Most dysgerminomas are seen in patients with a normal karyotype, but it is the most frequent ovarian neoplasm in patients with gonadal dysgenesis. About 5% to 10% are associated with gonadoblastomas in sexually maldeveloped patients. Most dysgerminomas occur in normal females who usually present with abdominal enlargement, a mass, or pain due to torsion. About 10% of dysgerminomas are bilateral on gross examination and another 10% have microscopic involvement of the contralateral ovary. Association with gonadoblastoma increases the risk of bilateral involvement by dysgerminoma.
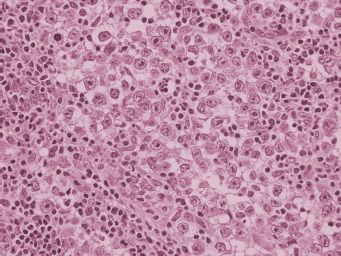
On gross examination, dysgerminomas are usually large, white to gray, fleshy lobulated masses that have no more than very focal areas of hemorrhage or necrosis on cut section. Abundant hemorrhage, necrosis, or cystic areas in a well-fixed tumor should raise the question of a mixed germ cell tumor. Microscopically, dysgerminomas display nests and cords of primitive-appearing germ cells with clear to eosinophilic cytoplasm and prominent cytoplasmic borders (Fig. 25.1). Nuclei are enlarged but they are not pleomorphic. Mitoses may be numerous, and the number of mitotic figures does not have any therapeutic or prognostic significance. Nests of tumor cells are separated by fibrous trabeculae that contain lymphocytes and, sometimes, granulomas. Syncytiotrophoblast cells are present in about 3% of dysgerminomas.
Dysgerminomas contain cytoplasmic glycogen that can be demonstrated with periodic acid Schiff (PAS) stain. These tumors display diffuse staining for placenta-like alkaline phosphatase (PLAP), usually with accentuation of the cytoplasmic membrane. Positive staining for c-kit (3) and the nuclear transcription factor OCT 3/4 (4) are helpful in confirming this diagnosis, dysgerminoma being the only OGCT that displays c-kit staining (5). Approximately one-third of dysgerminomas harbor c-kit amplifications or activating mutations, and these molecular alterations correlate with advanced stage (6). Both dysgerminoma and embryonal carcinoma stain for OCT 3/4. In contrast to embryonal carcinoma, dysgerminoma does not stain for CD30. Syncytiotrophoblast cells present in dysgerminomas display human chorionic gonadotropin (HCG) staining. In contrast to choriocarcinoma, the syncytiotrophoblast cells in dysgerminomas are not admixed with cytotrophoblast cells. Dysgerminomas do not produce alpha-fetoprotein (AFP), a finding that may be helpful in distinguishing them from the solid variant of yolk sac tumor. Optimal and prompt fixation of the surgical specimen facilitates the correct diagnosis. Poor fixation can result in artifacts that mimic embryonal carcinoma and yolk sac tumor histology.
Yolk Sac Tumor
Yolk sac tumor (endodermal sinus tumor) is the second most common OGCT, accounting for 22% of cases studied at the Armed Forces Institute of Pathology (AFIP) (2, 7). These tumors grow very rapidly, often becoming clinically evident in less than 1 month.
Ovarian yolk sac tumors are typically large and unilateral, although metastasis to the opposite ovary may occur. These tumors have a smooth external surface unless rupture or invasion into surrounding structures has occurred. On cut section, these neoplasms are tan to gray, with abundant hemorrhage and necrosis. They may be partially solid, but they usually contain cysts that vary in size from a few millimeters to several centimeters in diameter. The cut surface appears mucoid, slimy, or gelatinous. Yolk sac tumor (7) display many different histologic patterns (8). The most common microscopic pattern in primary ovarian tumors is the reticular or microcystic pattern (Fig. 25.2). The tumor has a mesh-like pattern and it displays a network of flattened or cuboidal epithelial cells with varying degrees of atypia. The endodermal sinus (festoon) pattern contains Schiller-Duval bodies (Fig. 25.3) that have a central capillary surrounded by connective tissue and a peripheral layer of columnar cells. These structures are situated in cavities lined by yolk sac tumor cells. When present, Schiller-Duval bodies are diagnostic of yolk sac tumor. Other less common variants of yolk sac tumor include hepatoid, polyvesicular vitteline, enteric, endometrioid, solid, parietal, and mesenchymal patterns. Most patterns of yolk sac tumor may contain eosinophilic hyaline globules that are PAS positive and diastase resistant. These globules may be seen in non-germ cell tumors and they are not specific for yolk sac tumor. They do not contain AFP. Yolk sac tumors generally display cytoplasmic staining for cytokeratin and AFP, although the parietal pattern of yolk sac tumor typically does not contain AFP. Therefore, serum AFP is a useful marker for yolk sac tumor, although a negative serum AFP does not exclude the disease. Chemotherapy has resulted in the appearance of AFP-negative parietal yolk sac tumor after eradication of AFP-positive patterns of the tumor (9). Enteric glands in yolk sac tumor may be carcinoembryonic antigen (CEA)-positive. Some types of yolk sac tumor need to be differentiated from endometrioid and clear cell tumors of the ovary. Germ cell tumors usually occur in younger patients than epithelial ovarian tumors, but the lack of staining for cytokeratin 7 and epithelial membrane antigen supports a diagnosis of yolk sac tumor (10) in controversial neoplasms.
FIGURE 25.1. Dysgerminoma. This neoplasm has nests of cells with clear cytoplasm and enlarged hyperchromatic nuclei. Fibrous septae containing lymphocytes separate nests of tumor.
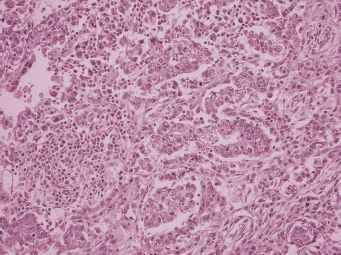
FIGURE 25.2. Reticular pattern of yolk sac tumor. There is a mesh-like arrangement of cuboidal tumor cells.
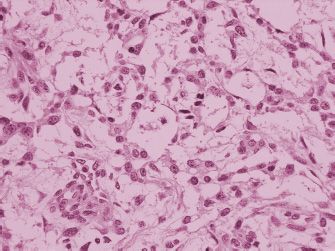
FIGURE 25.3. Schiller-Duval bodies, papillary structures with central blood vessels, are seen in the endodermal sinus pattern of yolk sac tumor.
Embryonal Carcinoma
Embryonal carcinoma is rarely seen in the ovary, in contrast to its frequent occurrence in the testis. Only 14 cases were identified during a period of 30 years at the AFIP (11), and there have been no recent large series of these tumors. Embryonal carcinoma of the ovary is usually associated with yolk sac tumor in mixed germ cell tumors. On gross examination, embryonal carcinoma characteristically displays areas of hemorrhage and necrosis. Microscopically, this tumor is composed of very crowded cells that display overlapping nuclei in paraffin sections. The nuclei are very pleomorphic and they contain large, prominent nucleoli. The mitotic rate is high in these tumors. Glandular, solid, and papillary patterns may be seen. Vascular invasion is common. Embryonal carcinoma stains positively for PLAP, pan-cytokeratin (AE1/AE3 and CAM 5.2), CD30, and OCT3/4. In contrast to seminoma, embryonal carcinoma does not display c-kit staining. Some embryonal carcinomas display focal AFP positivity that may represent partial transformation to yolk sac tumor. Syncytiotrophoblast cells may be present. They produce hCG, but they are not accompanied by admixed cytotrophoblast cells unless choriocarcinoma is also present.
Polyembryoma
Polyembryoma is a very rare malignant ovarian tumor (2). In the few cases reported, the embryoid bodies characteristic of this germ cell tumor have coexisted with other germ cell tumor types. The microscopic appearance of embryoid bodies (12), with an embryonic disc separating a yolk sac and an amniotic cavity, may actually be due to an admixture of yolk sac tumor and embryonal carcinoma.
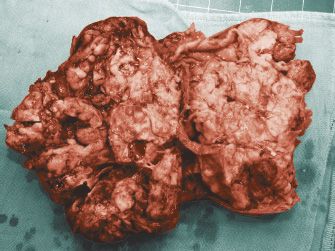
FIGURE 25.4. Choriocarcinoma. This ovarian neoplasm is extremely hemorrhagic.
Choriocarcinoma
Primary nongestational ovarian choriocarcinoma is rare (13). It is most often seen as a component of mixed germ cell tumors of the ovary (14,15). Choriocarcinomas display abundant hemorrhage and necrosis on gross examination (Fig. 25.4). Microscopically, these neoplasms show a plexiform pattern composed of an admixture of syncytiotrophoblast and cytotrophoblast cells (Fig. 25.5). Syncytiotrophoblastic giant cells have abundant eosinophilic to amphophilic cytoplasm that contains multiple atypical, hyperchromatic nuclei. Cytotrophoblast cells are round and often have well-defined cell borders, clear to lightly eosinophilic cytoplasm, and single, atypical nuclei. Numerous mitoses are present. Choriocarcinoma spreads by blood vessel invasion that is easy to identify in these tumors. Cytotrophoblast cells do not produce hCG. Syncytiotrophoblast cells are formed from cytotrophoblast cells, and syncytiotrophoblast does produce hCG. Choriocarcinoma may also stain for cytokeratins, epithelial membrane antigen, and carcinoembryonic antigen. Nongestational choriocarcinoma must be distinguished from gestational choriocarcinoma because the former has a worse prognosis and requires more aggressive therapy. Identification of paternal genetic material indicates that the tumor is of gestational origin (16).
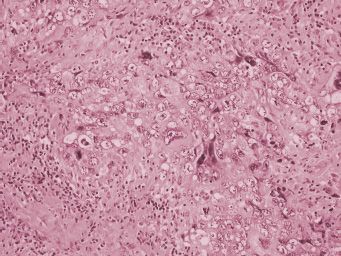
FIGURE 25.5. Choriocarcinoma. Syncytiotrophoblast cells have dark cytoplasm and multiple atypical nuclei. Cytotrophoblast cells have lighter cytoplasm and single atypical nuclei. Both cell types are admixed in choriocarcinoma. Areas of hemorrhage and necrosis are common.
Mixed Germ Cell Tumors
Mixed germ cell tumors of the ovary contain 2 or more different types of germ cell neoplasm, either intimately admixed or as separate foci within the tumor (14,15). They are much less common in the ovary than in the testis. They accounted for only 8% of malignant ovarian germ cell tumors accessioned at the AFIP over a period of 30 years (14).
Malignant mixed germ cell tumors are large, unilateral neoplasms, but the gross appearance on the cut surface depends on the particular types of germ cell tumor present. The most common germ cell tumor element in the AFIP series was dysgerminoma (80%), followed by yolk sac tumor (70%), teratoma (53%), choriocarcinoma (20%), and embryonal carcinoma (13%) (14). The most frequent combination has been dysgerminoma and yolk sac tumor. Syncytiotrophoblast may occur either as a component of choriocarcinoma or as isolated cells in other germ cell tumor elements. The diagnosis and prognosis of malignant mixed germ cell tumors depend on adequate tumor sampling to detect small areas of different types of germ cell tumor. Thorough sampling is essential because the types of tumor identified may affect therapy and prognosis.
Teratomas
Teratomas are germ cell tumors that contain tissue derived from 2 or 3 embryonic layers. Teratomas are subclassified according to whether the tumor elements represent mature or immature tissue types. In addition, monodermal and highly specialized teratomas are composed of a predominance of one tissue type such as thyroid tissue (struma ovarii). This discussion of the pathology of ovarian tumors will address predominantly mature and immature teratomas in adult women. In contrast to other ovarian germ cell tumor types, ovarian teratomas do not contain 12p amplification (17,18). Ovarian teratomas apparently arise by parthenogenesis.
Most teratomas are mature cystic teratomas that contain differentiated tissue components such as skin, cartilage, glia, glandular elements, and bone. Any tissue type present in adults may be represented in teratomas. The widest variety of tissue types is characteristically identified in a nodule in the wall of the cystic neoplasms. Mature cystic teratomas represent benign neoplasms unless they contain a somatic malignancy such as squamous carcinoma, papillary thyroid carcinoma, or other non-germ cell tumors arising in differentiated elements of the teratoma.
Immature teratomas in adult women, in contrast to mature cystic teratomas, are uncommon tumors. They represent about 3% of all ovarian teratomas, but immature teratomas are the third most common form of malignant ovarian germ cell tumors. Very limited amounts of immature tissue occurring in mature cystic teratomas do not seem to alter the prognosis of those tumors (19), but immature tissue in solid teratomas represents a malignant tumor that can disseminate and metastasize.
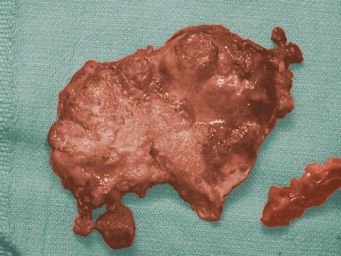
Most immature ovarian teratomas are unilateral neoplasms, although they can metastasize to the opposite ovary and they can also be associated with mature teratoma in the opposite ovary. Immature teratomas are predominantly solid tumors, but they may contain some cystic areas. The cut surface of immature teratomas is soft and fleshy or encephaloid in appearance (Fig. 25.6). Areas of hemorrhage and necrosis are common. Microscopically, these tumors contain a variety of mature and immature tissue components. The immature elements almost always consist of immature neural tissue in the form of small round blue cells focally organized into rosettes and tubules (Fig. 25.7). There is a correlation between disease prognosis and the degree of immaturity in the teratoma. The 3-tiered grading system is still the one most often used (20). Grade 1 neoplasms display some immaturity, but the immature neural tissue does not exceed, in aggregate, the area of one low-power field (40X) in any slide. Grade 2 teratomas contain more immaturity, but immature neural tissue occupies no more than an area equal to 3 low-power fields in any slide. Grade 3 neoplasms contain immature neural tissue that occupies an area greater than 3 low-power fields in at least one slide (21). Mature tissue is easily identified in grade 1 tumors. It is present to a lesser extent in grade 2 neoplasms and may be absent altogether in grade 3 immature teratomas. The amount of mitotic activity and immature neural tissue with rosettes and tubules also increases with increasing tumor grade. Some authors prefer classifying immature teratomas as either low (grade 1) or high (grades 2 and 3) grade teratomas. In patients whose neoplasm has disseminated beyond the ovary, the grade of the tumor metastasis is important in predicting survival and determining treatment. Occasionally, patients may have peritoneal implants that contain only mature tissue, but these mature glial implants may represent host tissue and not actual tumor implants (22). It is extremely important to sample peritoneal disease thoroughly in order to identify foci of immature teratoma.
FIGURE 25.6. Ovarian immature teratoma. The neoplasm is predominantly solid and has a soft, encephaloid appearance.
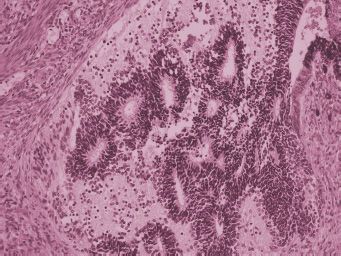
FIGURE 25.7. Ovarian immature teratoma. Immature neural tissue forms tubules.
A rare, but distinct entity deserves mention. The poorly differentiated small cell carcinoma of the ovary, classically associated with hypercalcemia (23) may have germ cell origin, as suggested by immunostaining for α-1-antitrypsin, presence of PAS-positive intracellular globules, presence of foci of intercellular basement membrane material, and focal laminin immunoreactivity (24). Microscopically, the small cell carcinoma of the ovary has similar features to that of the lung. Neuroendocrine differentiation is present with typical growth pattern, evidence of secretory granules, and expression of neuroendocrine markers; synapthophysin and chromogranin (25). These tumors occur usually in young women and are invariably associated with a dismal prognosis (26,27). Rapidly enlarging abdominal or pelvic mass is a common manifestation. Hypercalcemia is present in two-thirds of cases and small cell carcinoma is the most common cause of ovarian tumor–associated hypercalcemia. Because of its aggressive course, some women have symptoms of overt metastatic disease at presentation. Treatment with germ cell type chemotherapy regimen is probably justified, although data are scant and inconclusive, given the rarity of such tumors.
Biologically, ovarian germ cell tumors, like testis cancer, are derived from primordial germ cells, which undergo defective meiosis. Karyotypic abnormalities are common and include aneuploidy or chromosomal rearrangements. In contrast, benign teratomas have a normal karyotype. One report notes chromosomal abnormalities in 7% of mature teratomas (28,29). Analysis of centromeric heteromorphism suggests that 65% to 70% of benign teratomas result from a post-meiosis I type error (homozygotes), while the remaining 30% to 35% are caused by defective meiosis I, as demonstrated by heterozygosity of centromeric markers (28). Among malignant ovarian germ cell tumors, aneuploidy and chromosomal translocations or truncations similar to those encountered in testicular carcinoma have been widely reported (30,31). The presence of an isochromosome 12p (i12p) has been noted in ovarian tumors (32), albeit less commonly than in testis cancer. Other chromosomal aberrations such as loss or gain in chromosomes 1, 11, 12, 16, and X can be identified (33). The association between dysgerminoma and dysgenetic gonads (34) is well recognized and should be managed accordingly, as will be discussed.
Clinical Features
Malignant germ cell tumors of the ovary occur mainly in girls and young women.
In the University of Texas M.D. Anderson Cancer Center (UTMDACC) series, the age of the patients ranged from 6 to 40 years, with a median age of 16 to 20 years, depending upon histological type (35). There are ethnic and racial differences in the incidence of germ cell tumors, as shown in an analysis based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, with increased incidence of OGCTs among pediatric Black females compared to Black males and among Hispanic girls aged 10 to 19 years, as compared to non-Hispanics (36). Interestingly, a case-cohort study from the Children Oncology Group, including 274 cases (195 OGCTs and 79 testicular cancers), showed that a family history of cancer was inversely correlated with the risk of developing germ cell tumors (37).
Signs and symptoms in these patients are rather consistent. Abdominal pain associated with a palpable pelvic-abdominal mass is present in approximately 85% of patients (15,38,39). Ten percent of patients present with acute abdominal pain, usually caused by rupture, hemorrhage, or ovarian torsion. This finding is somewhat more common in patients with endodermal sinus tumor or mixed germ cell tumors and is frequently misdiagnosed as acute appendicitis. Less common signs and symptoms include abdominal distention (35%), fever (10%), and vaginal bleeding (10%). A few patients exhibit isosexual precocity, presumably due to hCG production by tumor cells.
In a small percentage of cases, ovarian germ cell tumors occur during pregnancy or in the immediate postpartum period (38). In the series reported by Gordon, 20 of 158 patients with dysgerminoma were diagnosed during pregnancy or after delivery (40). Nondysgerminomatous ovarian tumors occur less frequently during pregnancy, but rare cases have been reported (41–44). Marked increase in AFP heralds the presence of a germ cell tumor with yolk sac component. By and large, patients with ovarian tumors diagnosed during pregnancy can be treated successfully, without compromising the health of the fetus. Surgical resection of tumors and chemotherapy have been performed safely in mid and third trimesters. However, rapid disease progression and/or pregnancy termination/miscarriage have been recorded, especially for nondysgerminomatous tumors (45).
Many germ cell tumors possess the unique property of producing biologic markers that are detectable in serum. The development of specific and sensitive radioimmunoassay techniques to measure hCG and AFP led to dramatic improvements in patient monitoring. Serial measurements of serum markers aid the diagnosis and, more importantly, are useful for monitoring response to treatment and detection of subclinical recurrences. Table 25.1 illustrates typical findings in the sera of patients with various tumor histologic types. Endodermal sinus tumor and choriocarcinoma are prototypes for AFP and hCG production, respectively. Embryonal carcinoma can secrete both hCG and AFP, but most commonly produces hCG. Mixed tumors may produce either, both, or none of the markers, depending on the type and quantity of elements present. Dysgerminoma is commonly devoid of hormonal production, although a small percentage of tumors produces low levels of hCG, if multinucle-ated syncytiotrophoblastic giant cells are present. The presence of an elevated level of AFP or high level of hCG (>100 Units/mL) denotes the presence of tumor elements other than dysgerminoma. Therapy should be adjusted accordingly (see below). Although immature teratomas are associated with negative markers, a few tumors can produce AFP. A third tumor marker is lactic dehydrogenase (LDH), which is frequently elevated in patients with dysgerminoma or other germ cell tumors. Unfortunately, it is less specific than hCG or AFP, which limits its usefulness. CA-125 also can be nonspecifically elevated in patients with ovarian germ cell tumors (46). Age over 45 years, stage greater than I, and yolk sac tumor histology have been identified as prognostic factors that affect survival (47).
Serum Tumor Markers in Malignant Germ Cell Tumors of the Ovary |
Histology | AFP | hCG |
Dysgerminoma | − | ± |
Endodermal sinus tumor | + | − |
Immature teratoma | ± | − |
Mixed germ cell tumor | ± | ± |
Choriocarcinoma | − | + |
Embryonal carcinoma | ± | + |
Polyembryoma | ± | + |
SURGERY
Operative Findings
Malignant germ cell tumors of the ovary tend to be quite large. In the UTMDACC series, these tumors ranged in size from 7 to 40 cm, with a median size of 16 cm (15). Predominance of right-sided over left-sided involvement was noted. Bilaterality of tumor involvement (especially true stage IB disease) is exceedingly rare, except for dysgerminoma. Bilateral involvement occurs in 10% to 15% of dysgerminoma patients (40,48–51). For nondysgerminomatous tumors, bilateral involvement signifies either advanced disease with metastatic spread to the contralateral ovary or the presence of a mixed germ cell tumor with prominent dysgerminoma component.
Ascites may be noted in approximately 20% of cases. Rupture of tumors, either preoperatively or intraoperatively, can occur in approximately 20% of cases. Torsion of the ovarian pedicle was documented in 5% of patients in the UTMDACC series.
Benign cystic teratoma is associated with malignant germ cell tumors in 5% to 10% of cases. These coexistent teratomas may occur in the ipsilateral ovary, in the contralateral ovary, or bilaterally. Likewise, a preexisting gonadoblastoma may be noted in association with dysgerminoma and dysgenetic gonads related to a 46,XY karyotype (34,52–54).
Malignant germ cell tumors generally spread in 1 of 2 ways: along the peritoneal surface or through lymphatic dissemination. Although the relative frequency of these 2 principal mechanisms is difficult to discern, it is generally accepted that these neoplasms more commonly metastasize to lymph nodes than epithelial tumors. The high prevalence of inadequate staging procedures makes the true incidence of lymph node involvement uncertain. It is our impression that although still uncommon, malignant germ cell tumors have a somewhat greater predilection than epithelial tumors to metastasize hematogenously to parenchyma of liver or lung. The stage distribution is also very different from that of epithelial tumors. In most large series, approximately 60% to 70% of tumors will be stage I (40). The next most common stage is III, accounting for 25% to 30% of tumors. Stages II and IV are relatively uncommon.
Extent of Primary Surgery
The initial treatment approach for a patient suspected of having a malignant ovarian germ cell tumor is surgery, both for diagnosis and for therapy. After an adequate vertical midline incision, a thorough determination of the disease extent by inspection and palpation should be made. If the disease is confined to one or both ovaries, it is imperative that proper staging biopsies be performed (see below).
The type of primary operative procedure depends upon the surgical findings. Because many of these patients are young women, for whom preservation of fertility is a priority, minimizing the surgical resection while ensuring removal of tumor bulk must be thoughtfully balanced. As noted previously, bilateral ovarian involvement is rare, except for the case of pure dysgerminoma. Bilateral involvement may be found in cases of advanced disease (stages II–IV), in which there is metastasis from one ovary to the opposite gonad, or in cases of mixed germ cell tumors with dysgerminoma component. Therefore, fertility-sparing unilateral salpingo-oophorectomy with preservation of the contralateral ovary and of the uterus can be performed in most patients (55–57). If the contralateral ovary appears grossly normal on careful inspection, it should be left undisturbed. However, in the case of pure dysgerminoma, biopsy may be considered, because occult or microscopic tumor involvement occurs in a small percentage of patients. Unnecessary biopsy, however, may result in future infertility due to peritoneal adhesions or ovarian failure. If the contralateral ovary appears abnormally enlarged, a biopsy or ovarian cystectomy should be performed. If frozen examination reveals a dysgenetic gonad, or if there are clinical indications suggesting a hermaphrodite phenotype, then bilateral salpingo-oophorectomy is indicated. However, it is difficult to establish this diagnosis on frozen section. This determination should preferably be made by determining a normal female karyotype preoperatively. If benign cystic teratoma is found in the contralateral ovary, an event that can occur in 5% to 10% of patients, then ovarian cystectomy with preservation of remaining normal ovarian tissue is recommended.
An important problem, albeit rare, is bilateral gonadal involvement in a patient who desires to preserve fertility and who is a candidate for postoperative chemotherapy. There are no data regarding the ability of chemotherapy to eradicate a primary ovarian tumor. In testis cancer, there are presumptive data suggesting that tumor may persist after chemotherapy in the gonad and that the testis may be a drug sanctuary. In exceptional situations, it may be reasonable to preserve an involved ovary in a patient who will be receiving chemotherapy. However, it is conceivable that ovarian preservation could increase the risk for recurrence in these selected cases. The decision to preserve an involved ovary is difficult and must be made carefully considering patient’s wishes.
The advent of in vitro fertilization technology also has an impact on operative management (58). Convention has dictated that if a bilateral salpingo-oophorectomy is necessary, a hysterectomy should also be performed. However, with current assisted reproduction technologies (ART) involving donor oocyte and hormonal support, a woman without ovaries could potentially sustain a normal intrauterine pregnancy. Similarly, if the uterus and one ovary are resected because of tumor involvement, current techniques provide the opportunity for oocyte retrieval from the remaining ovary, in vitro fertilization with sperm from her male partner, and embryo implantation into a surrogate’s uterus. As the field of ARTs is evolving, traditional guidelines concerning surgical treatment in young patients with gynecologic tumors have to be thoughtfully adapted to individual circumstances.
Surgical Staging
Surgical staging information is essential for determining extent of disease, providing prognostic information, and guiding postoperative management. A meticulous approach is important for every patient, but is of critical importance for those patients with early clinical disease to detect the presence of occult or microscopic metastases. Staging of ovarian germ cell tumors follows the same principles applicable to epithelial ovarian tumors, as described by the International Federation of Gynecologists and Obstetricians (FIGO); see Table 25.2. Proper staging procedures consist of the following:
1. Although a transverse incision is cosmetically superior, a vertical midline incision is usually necessary for adequate exposure, appropriate staging biopsies, and resection of large pelvic tumors or metastatic disease in the upper abdomen.
2. Ascites, if present, should be evacuated and submitted for cytologic analysis. If no peritoneal fluid is noted, cytologic washings of the pelvis and bilateral paracolic gutters should be performed prior to manipulation of the intraperitoneal contents.
FIGO Staging of Ovarian Germ Cell Tumors |
Stage | Description |
I | Tumor limited to ovaries. |
IA | Tumor limited to one ovary, no ascites, intact capsule. |
IB | Tumor limited to both ovaries, no ascites, intact capsule. |
IC | Tumor either stage IA or IB, but with ascites present containing malignant cells or with ovarian capsule involvement or rupture or with positive peritoneal washings. |
II | Tumor involving one or both ovaries with extension to the pelvis. |
IIA | Extension to uterus or tubes. |
IIB | Involvement of both ovaries with pelvic extension. |
IIC | Tumor either stage IIA or IIB, but with ascites present containing malignant cells or with ovarian capsule involvement or rupture or with positive peritoneal washings. |
III | Tumor involving one or both ovaries with tumor implants outside the pelvis or with positive retroperitoneal or inguinal lymph nodes. Superficial liver metastases qualify as stage III. |
IIIA | Tumor limited to the pelvis with negative nodes but with microscopic seeding of the abdominal peritoneal surface. |
IIIB | Negative nodes, tumor implants in the abdominal cavity <2 cm. |
IIIC | Positive nodes or tumor implants in the abdominal cavity >2 cm. |
IV | Distant metastases present. |
3. The entire peritoneal cavity and its structures should be carefully inspected and palpated in a methodical manner. We generally prefer to start with the subphrenic spaces and move caudad toward the pelvis. The subdiaphragmatic areas, omentum, colon, all peritoneal surfaces, the entire retroperitoneum, and small intestinal serosa and mesentery should be checked. If any suspicious areas are noted, they should be submitted for biopsy or excised.
4. Next, the primary ovarian tumor and pelvis should be examined. Both ovaries should carefully be assessed for size, presence of obvious tumor involvement, capsular rupture, external excrescences, or adherence to surrounding structures.
5. If disease seems to be limited, that is, confined to the ovary or localized to the pelvis, then random staging biopsies of structures at risk should be performed. These sites should include the omentum (with generous biopsies from multiple areas) and the peritoneal surfaces of the following sites: bilateral paracolic gutters, cul-de-sac, lateral pelvic walls, vesicouterine reflection, and subdiaphragmatic areas. Any adhesions should also be generously sampled.
6. The paraaortic and bilateral pelvic lymph node-bearing areas should be carefully palpated. Any suspicious nodes should be excised or sampled. If no suspicious areas are detected, these areas should be sampled. There is no evidence that a complete paraaortic and/or pelvic lymphadenectomy is advantageous.
7. If obvious gross metastatic disease is present, it should be excised if feasible, or at least sampled to document disease extent. The concept of cytoreductive surgery is discussed below.
The gynecologic literature is replete with examples of inadequate surgical staging. The assumption that surgical staging in the 1990s is superior to that of several years ago may be erroneous. Most patients still undergo initial surgery in community hospitals and are inadequately staged. Upon referral of such a patient to a university or tertiary care center, the oncologist is faced with the dilemma of inadequate staging information. In such cases, postoperative studies including computed tomography of the abdomen are recommended. If histopathologic and limited anatomic information from the first surgery clearly indicates the use of systemic chemotherapy, it is generally inadvisable to consider re-exploration solely for the purpose of precise staging information. Reoperation to complete comprehensive staging may be appropriate under clinical circumstances where careful surveillance observation after complete staging is a sensible alternative to chemotherapy.
Cytoreductive Surgery
If widely spread tumor is encountered at initial surgery, it is recommended that the same principles concerning cytoreductive surgery applied in the surgical management of advanced epithelial ovarian cancer be followed. Specifically, as much tumor as is technically feasible and safe should be resected. However, because of their rarity, there is scant information in the literature on the impact of cytoreductive surgery of malignant germ cell tumors.
In a study of the Gynecologic Oncology Group (GOG), Slayton et al. found that 15 of 54 (28%) patients with completely resected disease at primary surgery failed chemotherapy with a combination of vincristine, dactinomycin, and cyclophosphamide (VAC), as opposed to 15 of 22 (68%) patients with incompletely resected disease treated with the same regimen (59). Furthermore, a higher percentage of patients with bulky postoperative residual disease (82%) failed chemotherapy compared to those with minimal residual disease (55%). In a subsequent GOG study reported by Williams, patients received the combination regimen of cisplatin, vinblastine, and bleomycin (PVB). In this study, patients with nondysgerminomatous tumors and clinically nonmeasurable disease after surgery, had a greater likelihood of remaining progression-free than those with measurable disease (65% vs. 34%) (60). In addition, patients who had been surgically debulked to optimal disease had an outcome intermediate between patients with suboptimal disease and those with optimal disease without debulking.
Even with epithelial tumors, the relative influence of tumor biology, surgical skill, and aggressiveness remains uncertain. Germ cell tumors, especially dysgerminomas, are generally much more chemosensitive than epithelial ovarian tumors. Therefore, aggressive resection of metastatic disease in these cases, especially resection of bulky retroperitoneal nodes, is questionable. The surgeon must exercise thoughtful and mature intraoperative judgment when encountering such situations, carefully weighing the risks of cytoreductive maneuvers in the setting of chemosensitive tumors. There is no substitute for surgical experience and a clear understanding of the biological behavior of these neoplasms. Even in the face of extensive metastatic disease, it is possible to perform a fertility-sparing procedure with preservation of a normal contralateral ovary.
The value of secondary cytoreductive surgery in the management of malignant ovarian germ cell tumors is even less clear than that of primary cytoreductive surgery. Although secondary cytoreduction is of questionable benefit for patients with refractory epithelial ovarian cancer (61,62), germ cell tumors are relatively more chemosensitive than epithelial tumors and more likely to respond to second-line therapy. Therefore, if a patient has an isolated focus of persistent tumor after first-line chemotherapy in an area such as the lung, liver, retroperitoneum, or brain, then surgical extirpation should be considered before changing chemotherapy regimens. Although this clinical situation is extremely rare, it has been observed in other situations involving chemosensitive tumors, such as gestational trophoblastic disease and testicular cancer.
Unlike testicular cancer, the finding of a residual mass after completion of chemotherapy is less common in patients with ovarian germ cell tumors because these women are likely to have considerable tumor debulking at the time of the diagnostic surgical procedure and thus enter chemotherapy with significantly less tumor burden. At completion of chemotherapy, men with nonseminomatous tumors or seminoma may have persistent mature teratoma or desmoplastic fibrosis. In patients with bulky dysgerminoma, residual masses after chemotherapy are very likely to represent desmoplastic fibrosis. Although a number of patients with pure ovarian immature teratomas or mixed germ cell tumors have persistent mature teratoma at the completion of chemotherapy, as documented by second-look laparotomy (63), the majority are left with multiple small peritoneal implants rather than with a dominant mass. However, it is now recognized that occasional patients who have received chemotherapy for immature teratoma or mixed germ cell tumor containing teratoma will have bulky residual teratoma after chemotherapy. The natural history or biologic implications of this finding are not clear. In testicular cancer, patients with bulky residual teratoma may experience slow progression of tumor (64) or may develop overtly malignant tumors over time (65–68). There are similar anecdotal reports of progressive mature teratoma in ovarian germ cell tumor patients after chemotherapy (69–71). Considering this information, it seems appropriate to resect persistent masses in patients with negative markers after chemotherapy for germ cell tumors containing immature teratoma. If viable neoplasm is found, additional chemotherapy should be considered. However, if only mature teratoma is resected, observation is generally recommended.
Second-Look Laparotomy
Since 1960, second-look laparotomy was included in the routine management of patients with epithelial ovarian cancer to assess disease status after a fixed interval of chemotherapy. It was only natural that such an approach would be extrapolated to the management of patients with malignant ovarian germ cell tumors. In a review of the experience with second-look laparotomy at UTMDACC, findings were negative in 52 of 53 patients (72). The one patient with positive findings at second-look laparotomy had an elevated AFP level prior to surgery, which accurately predicted residual disease. This patient received subsequent chemotherapy with PVB, entering prolonged remission. Of the patients with negative findings, one woman relapsed 9 months after the negative second look surgery and subsequently died. Thirteen patients in this series had biopsy-proven evidence of residual mature teratoma (so-called “chemotherapeutic retroconversion”) at second-look laparotomy; treatment was discontinued in all patients and none developed recurrence. Thus, in this series, second-look surgery did not add prognostic information or alter the therapeutic management of patients. The role of second-look surgery is further obscured in the setting of advancement in imaging techniques (CT scanning, PET, and MRI) and in an era where tumor marker measurements are part of routine care of patients with germ cell tumors.
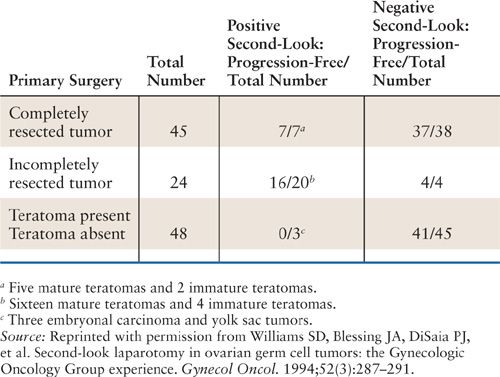
The GOG experience with second-look laparotomy in ovarian germ cell tumors has been reviewed (63). One hundred seventeen patients enrolled prospectively on 1 of 3 GOG protocols using cisplatin-based chemotherapy after initial surgical staging and cytoreduction (GOG protocols #45, 78, and 90) underwent second-look surgical procedures. Of these, 45 surgical procedures were performed in patients who received 3 courses of cisplatin, etoposide, and bleomycin (BEP) after complete tumor resection. In this subgroup, 38 patients had negative findings, 2 patients had immature teratoma, and 5 patients had mature teratoma. One of the patients with residual immature teratoma received further chemotherapy and one did not. Both these and the rest of patients were disease-free. One patient with negative second-look surgery findings subsequently relapsed and succumbed to disease. Hence, in the subgroup of patients with completely resected primary ovarian germ cell tumors, the benefit of second-look surgery is nil. In contrast, 72 patients in this series treated with similar chemotherapy had advanced incompletely resected tumor before beginning adjuvant treatment. In this subgroup, 48 patients did not have teratoma elements in their primary tumor. At second-look surgery, 45 patients had no residual tumor and 3 patients displayed persistent endodermal sinus tumor or embryonal carcinoma. All 3 of the latter patients died despite further treatment. Five patients with negative second laparotomies recurred, of which only one was salvaged with chemotherapy. Thus, the value of second look surgery in patients with incompletely resected germ cell tumors, not containing teratoma is arguably minimal. However, in the subgroup of patients with incompletely resected tumors containing teratoma elements (total of 24 patients), second-look surgery had an impact on subsequent management. Of these patients, 16 were found to have mature teratoma at second look, which was bulky or progressive in 7 cases. Four additional patients were found to have residual immature teratoma. Fourteen of the total 16 patients with teratoma and 6 of the 7 women with bulky residual tumor remained disease free after surgical resection. Therefore, while second-look laparotomy is not necessary in patients with tumor completely resected primarily or in those patients with initially incompletely resected tumor not containing teratoma, clinical benefit can be derived in those patients with incompletely resected primary tumor that contains elements of teratoma (see Table 25.3).
Advances in imaging technology, including the advent of positron emission tomography (PET scanning) may further obviate the need for surgical re-exploration. While PET scan is sensitive for detecting active (malignant) tumor, its usefulness in evaluating residual mature teratoma is more limited (73–76). A positive PET scan in the setting of a residual mass after treatment is highly indicative of viable tumor and when used in conjunction with traditional radiographic techniques (CT scan, MRI) and tumor marker determinations, can predict relapses with accuracy (77). A recent series demonstrates that in patients with residual masses after treatment for seminoma, a positive PET scan is strong evidence that the residual mass contains persistent tumor. In contrast, if the PET scan is negative, residual masses are unlikely to contain active tumor. The specificity of the PET scan in this situation was 100%, the sensitivity was 80%, and the positive and negative predictive values were 100% and 95%, respectively (78). Although studies using PET scanning in OGCT are scant (79,80), the concepts are very similar and can be extrapolated from the testis cancer literature.
Results of Second-Look Surgery in Patients Enrolled on GOG Protocols |
CHEMOTHERAPY FOR OVARIAN GERM CELL TUMORS
Chemotherapy: From VAC to PEB
One of the great triumphs of cancer treatment in the 1970s and 1980s has been the development of effective chemotherapy for testicular cancer (81,82). The lessons learned from prospective, randomized trials in testicular cancer subsequently have been applied to OGCTs. Presently, the overwhelming majority of patients with ovarian germ cell tumors survive their disease with the judicious use of surgery and cisplatin-based combination chemotherapy. There are many similarities, but a few important differences between testicular cancer and OGCTs.
Historically, the first regimens used successfully for women with OGCTs were VAC (vincristine, dactinomycin, and cyclophosphamide) or VAC-type regimens. Such treatment had curative potential, especially in early-stage disease. However, among patients with advanced disease, the number of long-term survivors after VAC therapy remained under 50%. In the series reported from UTMDACC, although 86% of patients with stage I tumors were cured with VAC, the efficacy of the regimen was significantly lower for patients with advanced disease (35). Only 57% of stage II patients and 50% of patients with stage III achieved long-term control. The 2 patients with stage IV tumors in this series succumbed to disease. Similarly, in a GOG study, only 7 out of 22 patients with incompletely resected OGCTs achieved long-term disease control after VAC, as compared to 39 of 54 patients with completely resected tumors (83). In that report, 11 of 15 patients with stage III and both patients with stage IV disease progressed within 12 months. These data suggest that VAC chemotherapy was insufficient for the treatment of advanced stage and/or incompletely resected OGCTs.
Because of the experience gained from the treatment of testicular germ cell tumors demonstrating the superiority of cisplatin-based regimens, new platinum-based regimens were tested in patients with ovarian germ cell tumors. Gershenson reported the efficacy of PVB in a small series of patients treated at UTM-DACC (84). Among 15 patients, 7 received PVB in the adjuvant setting and 9 received the combination at the time of recurrence. Six of 7 patients treated with PVB upfront became long-term survivors. Among them, 3 women had optimally debulked stage III disease.
Subsequently, the PVB combination was evaluated prospectively in GOG protocol #45 (60). In this study, 47 of 89 patients with nondysgerminomatous ovarian tumors (53%) were disease-free with a median follow-up of 52 months. The latest treatment failure occurred at 28 months. Eight other patients had durable remissions with second-line therapy and a few other patients had nonprogressive or slowly progressive immature teratoma. Thus, the 4-year overall survival was approximately 70%. Of note, 29% of patients enrolled in this trial had received prior radiation or chemotherapy, which might have affected negatively the overall outcome. As discussed previously, patients who were debulked to optimal disease fared better than those who were not. Histological type and marker elevation before treatment were not associated with adverse outcome. However, even among patients with nonmeasurable, and presumably small volume disease, and without prior treatment, 8 of 30 patients treated with PVB ultimately failed.
The BEP Regimena |
Cisplatin | 20 mg/m2 days 1–5 |
Etoposide (VP-16) | 100 mg/m2 days 1–5 |
Bleomycin | 30 units IV weekly |
a Three to 4 courses given at 21-day intervals.
In testicular cancer, subsequent experience has documented that etoposide is at least equivalent to vinblastine and produces improved survival in patients with high tumor volume (82). Furthermore, the use of etoposide in place of vinblastine led to reduced neurologic toxicity, abdominal pain, and constipation. The latter 2 adverse effects are particularly important for patients with OGCTs, as many would have had recent abdominal surgery. These observations led to the evaluation of the combination of BEP (Table 25.4) in patients with ovarian germ cell tumors. In a series from UTMDACC, long-term remissions were recorded in 25 of 26 patients treated with BEP (85). The only patient who succumbed to disease had been noncompliant with treatment, monitoring, and follow-up. In this series, 4 patients with measurable disease after surgery had complete remissions after BEP treatment. This led to a prospective GOG study evaluating BEP in patients with ovarian germ cell tumors (86). The regimen was highly effective, 91 of 93 enrolled patients being free of disease at follow-up. On the basis of these data, although BEP and VAC have not been prospectively compared, BEP emerged as the preferred regimen for patients with OGCTs. The inclusion of cisplatin in the treatment of ovarian tumors resulted indisputably in an improvement in survival and disease control, as shown by the results of GOG studies, as well as by other clinical series (87–89). These results of therapy are summarized in Table 25.5.
Differences in Outcome for Patients with Completely Resected Tumors Versus Advanced Stage Disease
It is clear that several prognostic factors impact the outcome of patients with OGCTs and that there are important differences between testicular and ovarian germ cell neoplasms. Debulking surgery plays a central role in the management of ovarian tumors, but has a less important role in testicular cancer. In the hands of an experienced surgeon, the majority of women with ovarian tumors are debulked to minimal and often clinically undetectable disease before starting chemotherapy. Therefore, unlike patients with testicular cancer, most women who are candidates for chemotherapy have minimal or no residual disease. However, even in this circumstance, there seems to be little doubt that adjuvant therapy is appropriate in the majority of cases. The anticipated risk of relapse with surgery alone in patients with advanced disease is as high as 75% to 80%. Particularly, patients with embryonal carcinoma, endodermal sinus tumors, and mixed tumors containing these elements are considered to be at very high risk of recurrence without postoperative therapy. This risk can be minimized by the use of adjuvant chemotherapy. In GOG protocol #78, 50 of 51 patients with completely resected OGCTs remained free of disease when 3 cycles of BEP were given adjuvantly. Other studies using cisplatin-based therapy have given similar results (Table 25.6). The recommended treatment for most patients (with the exception of patients with grade 1, stage IA immature teratoma, or stage IA dysgerminoma) is adjuvant chemotherapy with 3 courses of BEP. Virtually all patients with early stage or completely resected disease will survive after careful surgical staging and cisplatin-based adjuvant chemotherapy. More recently, clinical series and observations are beginning to suggest that surveillance with careful follow-up after surgery may be an acceptable alternative for carefully selected patients, as discussed below. Given the fact that surgery followed by chemotherapy is curative for most patients, this course of action should be taken only after very careful consideration. Further studies are needed in this area.
Adjuvant Chemotherapy |
Institution | Regimen | Progression-free/Total (%) |
GOG (66) | BEP | 89/93 (96) |
Australia (51) | Multiple | 9/10 (90) |
Hospital 12 de Octubre (32) | PVB or BEP | 9/9 (100) |
M.D. Anderson (18) | PVB | 4/4 (100) |
Instituto Nazionale Tumori (3) | PVB | 9/10 (90) |
M.D. Anderson (19) | BEP | 20/20 (100) |
Abbreviations: GOG, Gynecologic Oncology Group; BEP, cisplatin, etoposide, bleomycin; PVB, cisplatin, vinblastine, bleomycin.
In contrast, most clinical series have shown worse clinical outcome for patients with metastatic disease or with incompletely resected tumors (see Table 25.7). Current clinical trials in testicular cancer separate patients with small tumor volume and a resultant excellent prognosis from those with bulky tumor or liver and brain involvement (90). Patients in the former group are usually complete responders to chemotherapy and long-term survivors, whereas only about 50% to 60% of the latter patients are cured. Hence, clinical trials for patients with good prognostic factors investigate shorter or less toxic chemotherapy aiming at minimizing toxicity (91), while preserving efficacy. In contrast, clinical trials for patients with high-risk disease have evaluated more intensive chemotherapy regimens with the goal of improving the likelihood of cure (92,93). For instance, high-dose chemotherapy (HDCT) with stem cell rescue was tested for patients with high-risk testicular cancer in a multi-institutional clinical protocol (ECOG protocol 3894). Patients considered to have high risk for relapse were randomized to receive 4 cycles of BEP (control arm) versus 2 cycles of BEP followed by HDCT with autologous stem cell rescue in the form of 2 (tandem) courses using carboplatin, etoposide, and cyclophosphamide as a conditioning regimen (experimental arm). The 1-year durable complete response rate was 52% after BEP + HDCT and 48% after BEP alone (p = 0.53) (94). The results of the trial disproved the concept that more aggressive chemotherapy in the first-line setting improves outcome of high-risk testicular cancer patients when compared to standard dose BEP (95).
Chemotherapy of Advanced Disease |
Institution | Regimen | Progression-Free/Total (%) |
GOG (67) | PVB | 47/89 (53) |
Australia (51) | Multiple | 42/46 (91) |
Hospital 12 de Octubre (32) | PVB or BEP | 15/19 (79) |
M.D. Anderson (18) | PVB | 7/11 (64) |
Instituto Nazionale Tumori (13) | PVB | 7/14 (50) |
M.D. Anderson (19) | BEP | 5/6 (83) |
Note: BEP, bleomycin, etoposide, and cisplatin; GOG, Gynecologic Oncology Group; PVB, cisplatin, vinblastine, bleomycin.
Dependable risk stratification, as the one used for testicular tumors, is not currently in use for OGCTs. The only clinical prognosticators for outcome of OGCTs remain stage at diagnosis and increase in tumor marker levels (96). Whether this reflects an inherent biologic difference between ovarian germ cell tumors and testis cancer or merely an underestimation of tumor volume because of intraperitoneal spread is not clear. In summary, with the exception of patients with grade 1, stage IA immature teratoma, or stage IA dysgerminoma, the available evidence supports that women with OGCT should receive 3 to 4 cycles of BEP chemotherapy after cytoreductive surgery. Therapy courses longer than 4 cycles are not supported, regardless of tumor volume.
Management of Residual or Recurrent Disease
The large majority of patients with OGCTs are cured with surgery and platinum-based chemotherapy. However, a small percentage of patients have persistent or progressive disease during treatment or recur after completion of treatment. Like in testicular cancer, these treatment failures are categorized as platinum-resistant (progression during or within 4 to 6 weeks of completing treatment) or platinum-sensitive (recurrence beyond 6 weeks from platinum-based therapy).
Most recurrences occur within 24 months from primary treatment. In a series from UTMDACC, 42 treatment failures were identified among 160 patients with ovarian germ cell tumors treated between 1970 and 1990 (97). Treatment failure in these patients was attributed to inadequate surgery in 14 patients, inadequate radiation in 5 patients, inadequate chemotherapy in 16 patients (underdosing and noncompliance), treatment-related toxicity in 1 patient, and unidentifiable causes in 6 patients. A significant number of patients included in this series had received VAC-based chemotherapy, which accounted for the higher than expected rate of recurrence.
Given the high curability rate of OGCTs with primary treatment, the management of recurrent disease represents a complex and often difficult issue, and preferably should be performed in a specialized center. Data to guide the management of patients with recurrent OGCTs are scant and largely extrapolated from the clinical experience with testicular cancer. The single most important prognostic factor in patients with testicular cancer is whether or not they are refractory to cisplatin. The likelihood of cure with high-dose salvage therapy in patients who relapse from a complete remission after initial therapy is as high as 60% or more. On the other hand, in patients who are truly cisplatin-refractory, the likelihood of long-term survival and cure is significantly less. However, up to 30% to 40% of these patients can become long-term survivors. Approximately 30% of patients with recurrent platinum-sensitive testicular cancer can be salvaged with second-line chemotherapy (VeIP: vinblastine, ifos-famide, platinum) (92). However, there is now strong evidence that high-dose therapy with carboplatin, etoposide with or without cyclophosphamide or ifosfamide, and stem cell rescue is superior to standard dose salvage therapy for these patients (98,99). Generally, in patients who are not cisplatin-refractory one course of standard dose therapy, usually cisplatin, vinblastine, and ifosfamide, is given. If an initial response is seen, then 2 subsequent courses of high-dose chemotherapy (carboplatin and etoposide) with stem cell rescue are given (100). A recent report from Indiana University describes this approach among 184 patients with recurrent testicular cancer. At a median follow-up of 48 months, 116 patients were in complete remission. Remarkably, of the subgroup of 40 patients who were platinum refractory, 18 are disease-free after high-dose chemotherapy (101). While this approach has not been prospectively tested in women with recurrent platinum-sensitive OGCTs, because of the small numbers of patients, the concepts are very similar and support the use of high-dose therapy in this setting. Referral to a specialized center for management of recurrent disease is desirable.
Active agents in the setting of recurrence after high-dose chemotherapy include ifosfamide, taxanes, gemcitabine, and oxaliplatin (102–105). In a phase II trial from Indiana University, the combination of gemcitabine and paclitaxel induced objective responses in 10 of 31 patients who had recurred after high-dose chemotherapy. Of those, 5 patients were free of disease 2 years after treatment (105). The combination of gemcitabine and oxaliplatin (GemOx) induced a 46% response rate in a group of 31 patients with recurrent germ cell tumors. Over 60% of these patients were platinum-resistant or refractory (106). Referral for treatment with investigational agents for recurrent, refractory OGCTs is appropriate.
Immediate Toxicity of Chemotherapy
Acute adverse effects of chemotherapy can be substantial and these patients should be treated by physicians experienced in their management. About 25% of patients develop febrile neutropenic episodes during chemotherapy and require hospitalization and broad-spectrum antibiotics (107). Cisplatin can be associated with nephrotoxicity. This can be avoided by ensuring adequate hydration during and immediately after chemotherapy and by avoidance of aminoglycoside antibiotics. Bleomycin can cause pulmonary fibrosis (107). Pulmonary function testing is frequently used to follow these patients. However, the value of carbon monoxide diffusion capacity to predict early lung disease has been challenged (108). The most effective method for monitoring patients with germ cell tumors is careful physical examination of the chest. Findings of early bleomycin lung disease are a lag or diminished expansion of one hemithorax or fine basilar rales that do not clear with cough. These findings can be very subtle but if present immediate discontinuation of bleomycin should be mandated. It is important to note that randomized trials in good prognosis testicular cancer have suggested that bleomycin is an important component of the treatment regimen, particularly if only 3 courses of therapy are given (109,110). Other randomized trials have shown that carboplatin is inferior to cisplatin and cannot be substituted for cisplatin without worsening therapeutic outcome (111,112).
Patients with advanced OGCTs should receive 3 to 4 courses of treatment given in full dose and on schedule. There is presumptive evidence in testicular cancer that the timeliness of chemotherapy may be associated with outcome. Thus, treatment is given regardless of hematological parameters on the scheduled day of treatment. The impact of the hematopoietic growth factors (G-CSF, GM-CSF) on the management of the myelosuppressive complications of this chemotherapy has not been precisely defined. As most patients will not develop neutropenic fever or infection, hematopoietic growth factors are not routinely necessary (113). It is reasonable to use hematopoietic growth factors to avoid dose reductions for patients with previous episodes of neutropenic fever or in unusually ill patients who are at a higher risk of myelosuppressive complications, or those who received prior radiotherapy. Modern antiemetic therapy, an example of which is shown in Table 25.7, has greatly lessened chemotherapy-induced emesis. By following these guidelines and providing supportive care as indicated, virtually all patients can be treated on schedule, in full or nearly full dose. Chemotherapy-related mortality should be less than 1%. Indeed in GOG protocol #78, there were no toxic deaths among 93 patients treated. Late effects of chemotherapy are discussed below.
A Typical Antiemetic Regimen |
Granisetron 1 mg IV 30 min prior to cisplatin daily for 5 days
or
Ondanesietron 0.15 mg/kg IV 30 min prior and 4 h after cisplatin daily for 5 days
plus
Dexamethasone 20 mg IV 30 min prior to cisplatin on days 1 and 2
plus
Aprepitant 125 mg PO on day 1 and 80 mg PO on days 2 and 3, prior to cisplatin infusion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree