Figure 10.1 Incidence of invasive and borderline ovarian tumors in the United States by age. Source: Sherman ME, Berman J, Birrer MJ, et al. Current challenges and opportunities for research on borderline ovarian tumors. Hum Pathol. 2004;35:961–970.
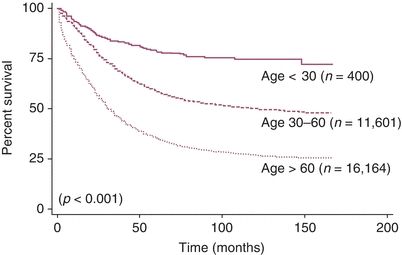
Figure 10.2 Disease-specific survival of patients based on age at diagnosis. Source: Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs. older women: A population-based analysis. Br J Cancer. 2006;95:1314–1320.
Table 10.1 Incidence of Stage and Grade by Age Grouping in SEER Data 1988 to 2001
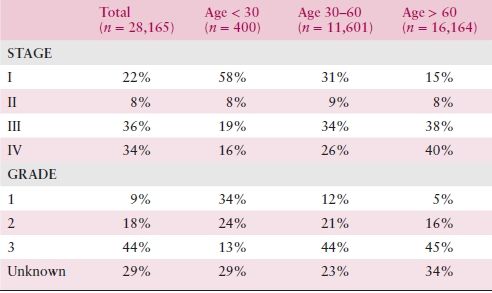
Source: Adapted from Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs. older women: A population-based analysis. Br J Cancer. 2006;95:1314–1320.
There have been no consistent relationships reported of specific dietary components to ovarian cancer risk. Although some studies had implicated a diet high in meat and animal fat or a diet high in lactose, most large recent studies have failed to demonstrate any relationship between the consumption of animal foods and the development of ovarian cancer. There does appear to be a modest inverse correlation between moderate physical activity and ovarian cancer risk, and obesity has consistently shown a positive association with ovarian cancer risk.
Case–control analyses have consistently documented that users of oral contraceptives (OCP) have a 30% to 60% decreased risk of developing ovarian cancer than do women who have never used OCP. Tubal ligation is also protective. Some reports have suggested an increased risk with use of fertility-inducing drugs such as clomiphene, but most recent studies have found either a weak association or no association between infertility treatment and development of ovarian cancer. There appears to be a modest association between hormone replacement therapy (HRT) and risk of ovarian cancer. Progestins have been proposed to be protective against ovarian cancer, and the risk of ovarian cancer may be greater for estrogen-only HRT than for estrogen–progestin combinations.
Approximately 15% to 20% of cases of invasive epithelial ovarian cancer are the result of autosomal dominant high-penetrant genetic factors, predominantly germline mutations in the BRCA1 or the BRCA2 genes. The BRCA1 and BRCA2 gene products function in the cellular response to DNA damage. The lifetime risk of ovarian cancer for women with BRCA1 mutations and BRCA2 mutations has been estimated to be 40% and 20%, respectively. Risk may be reduced by prophylactic salpingo-oophorectomy. Survival for women with ovarian cancer related to a BRCA1 or BRCA2 mutation is longer than for women with a similar stage cancer and no mutation. Issues related to BRCA mutations as well as mutations in genes involved in the mismatch repair pathway are discussed in Chapter 3. Epithelial ovarian cancer is also a component of Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer syndrome—HNPCC). In addition to a predisposition to develop colorectal and endometrial cancer, women with this syndrome have a 10% to 13% lifetime risk for developing ovarian cancer.
PATHOLOGY
KEY POINTS
- Most fatal ovarian cancers in the United States are high-grade serous neoplasms.
- Most serous ovarian cancers originate in the distal fallopian tube from serous tubal intraepithelial carcinoma (STIC) lesions.
- Borderline tumors are usually confined to the ovary and have a better prognosis.
- Primary mucinous ovarian cancers can be difficult to distinguish from metastases to the ovary from other sites.
Ovarian carcinoma includes many subtypes (Table 10.2). Approximately half of all ovarian tumors are of epithelial origin and account for 90% of malignant tumors. The vast majority of fatal ovarian cancers are high-grade serous carcinomas. Most recently, there have been new insights into the origin of serous “ovarian” cancer. A putative precursor lesion in the fallopian tube, STIC, has been identified, and it is now believed that most serous “ovarian” cancer originates in the distal fallopian tube as STIC lesions. However, it is often not possible to clearly identify where the serous tumor originated. This makes screening and early detection difficult. Most of the remaining cell types of ovarian carcinomas appear to have other precursor lesions. Nearly all clear cell carcinomas and a large proportion of endometrioid carcinomas arise in endometriosis and appear to progress through a hyperplasia–borderline tumor–carcinoma sequence. Similarly, mucinous carcinomas most probably transit through a mucinous cystadenoma–borderline tumor–intraepithelial carcinoma sequence before invasion occurs. Such tumors are much more likely to be diagnosed while still confined to the ovary, and they therefore comprise more curable ovarian carcinomas. Low-grade serous carcinoma is a recently described and relatively small subset of ovarian serous carcinoma that also often appears to follow a stepwise progression from borderline tumor to invasive carcinoma.
Table 10.2 Histologic Classification of Common Epithelial Tumors of the Ovary
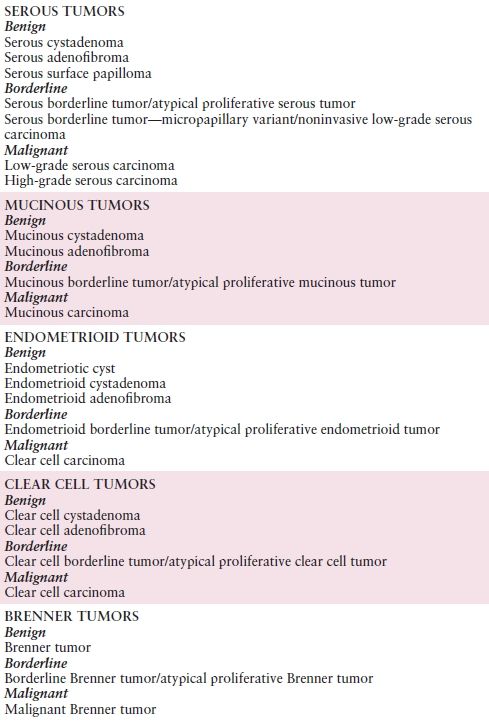
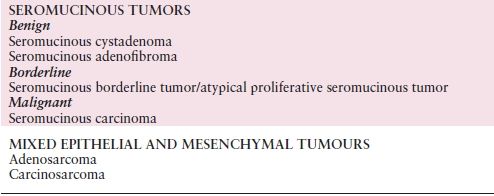
Source: Modified with permission from Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs, 4th ed. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2014:12–14.
Serous Tumors
Approximately 20% of serous tumors are malignant, 2% are borderline tumors, and 78% are benign. The mean age for patients with cancer is 56 years. Patients with benign and borderline tumors are generally younger, with mean ages at diagnosis of 45 and 48 years, respectively. Approximately 1 in 6 serous adenomas are bilateral compared to one-third of stage I serous adenocarcinomas. Those of higher stage are bilateral in two-thirds of cases. A recent Stanford series showed a 55% bilateral rate in serous borderline tumors. Borderline tumors that are confined to the ovary are associated with a survival that approaches 100%. The 10-year survival for women with tumors that have associated noninvasive peritoneal lesions still exceeds 95%, while for those with invasive implants it is 67%. Micropapillary serous tumors are a subtype of borderline tumors that are particularly likely to be associated with invasive implants and therefore have a worse prognosis. Microinvasion (defined variously as a maximal invasive focus size of 2, 3, or 5 mm, or an area of 10 mm2) can be found in up to 10% of borderline tumors. The overall prognosis for the patient with microinvasion in a borderline tumor is currently considered to be no different than that of a borderline tumor without microinvasion. Borderline serous tumors may also spread to lymph nodes, but this does not appear to worsen the overall good prognosis.
Serous adenocarcinomas range in size from small (2 to 3 cm) to quite large. They often present as solid masses bounded by a capsule, often containing areas of necrosis and hemorrhage. The ovary from which the neoplasm has arisen is frequently not apparent grossly or microscopically. The gross appearance of a typical high-grade serous carcinoma is not distinctive, and it may be mimicked by other high-grade epithelial ovarian neoplasms, granulosa cell tumors, and carcinomas metastatic to the ovary. The tumor may appear as large sheets of polygonal cells growing autonomously without stromal support or as broad to fine clusters of cells related to papillae that irregularly dissect through the stroma, accompanied by a host desmoplastic response. The nuclei are typically large and pleomorphic, with variably sized nucleoli and numerous mitotic figures. Two grading systems for serous carcinomas have been proposed and tested in the past decade: a 3-grade system and, more recently, a binary system of high-grade and low-grade serous carcinoma. In contrast to high-grade serous carcinoma, low-grade neoplasms are characterized by cells with only mild-to-moderate nuclear atypia, evenly distributed chromatin, variable nucleoli, and fewer than 10 mitoses/10 hpf. Micropapillary architecture is frequently observed. The binary system separates over 90% of advanced-stage serous carcinomas into the high-grade group. This distribution is reflected in the overall behavior of advanced-stage serous carcinoma, which is associated with a 5-year survival of approximately 25%. In contrast, the 5- and 10-year survivals for women with advanced-stage low-grade serous carcinoma are approximately 85% and 50% to 60%, respectively. Serous carcinomas frequently stain positively with immunohistochemistry for WT1 and cytokeratin (CK) 7, and negative or only focally positive for CK20 and calretinin. A panel that includes these antibodies is frequently employed to evaluate a carcinoma whose primary site is uncertain. Pancreatic and breast carcinomas have the same CK7/20 profile, and thus other markers are needed for these sites. GCDFP-15 (gross cystic disease fluid protein) is often positive in breast carcinoma and only rarely positive in ovarian carcinoma.
Mucinous Tumors
Primary ovarian mucinous tumors include three types: cystadenomas (81%; these are benign), borderline tumors (13%), and primary ovarian mucinous carcinomas (5%). The mean age for patients with mucinous adenocarcinoma is 52 years, which, as with serous adenocarcinoma, is greater than the mean age of patients with benign and borderline tumors (44 and 49 years, respectively).
Mucinous tumors can grow to extremely large sizes, being among the largest of any recorded tumor in the body. Sizes exceeding 40 kg and 30 cm in greatest diameter have been reported. There are two types of borderline mucinous tumors: the gastrointestinal type and the endocervical type. The gastrointestinal type is more common. Both types of borderline mucinous tumors are almost always stage I and have close to 100% survival. As with serous borderline tumors, the presence of microinvasion does not appear to affect the generally benign prognosis of these tumors.
Primary ovarian mucinous carcinomas are typically unilateral multicystic mucinous masses with smooth capsules. They may contain solid areas and regions of necrosis, and rupture with surface involvement can occur. A thick tenacious mucinous material may fill the cysts. Most are stage I cancers. Pseudomyxoma peritonei is a clinicopathologic syndrome in which mucinous ascites is accompanied by low-grade neoplastic mucinous epithelium intimately associated with pools of extracellular mucin and fibrosis. It was thought that this syndrome sometimes resulted from mucinous ovarian tumors, but now it is believed to be derived universally from appendiceal low-grade (adenomatous) mucinous tumors; the ovarian involvement is secondary.
Mucinous carcinomas with secondary spread to the ovary are much more commonly encountered than primary ovarian mucinous carcinomas, and the distinction can be difficult to make. The ovarian metastasis may be the presenting site of disease. Approximately 80% of mucinous carcinomas that involve the ovary are metastatic from other sites. Metastatic mucinous carcinomas are usually readily recognized as such when the ovarian tumors exhibit any or all of the following features: bilateral, smaller size (typically <10 cm), ovarian surface involvement, a nodular pattern of involvement, and an infiltrative pattern of stromal invasion. However, some metastatic mucinous carcinomas, especially those derived from the colorectum, pancreaticobiliary tract, appendix, and endocervix, can exhibit deceptive patterns of invasion. Immunohistochemical analysis with antibodies such as CK7, CK20, CDX-2, and p16 can be useful for identifying some metastatic mucinous carcinomas; however, the utility of currently available markers is limited due to overlapping immunoprofiles of primary ovarian mucinous tumors with other mucinous tumors, particularly those of upper gastrointestinal tract origin.
Endometrioid Tumors
Endometrioid tumors account for a relatively small proportion of the common epithelial tumors, but most endometrioid tumors are malignant. When controlled for stage, the survival rate for endometrioid tumors is similar to that of serous adenocarcinoma in some studies but better in others. About 10% to 40% of cases are associated with endometriosis. Approximately 14% of women with endometrioid ovarian carcinoma also have endometrial cancer of the uterine corpus. Endometrial hyperplasia is also commonly present. The similar histology and subtype and high survival rate of these patients (80% at 10 years) suggest that the majority are synchronous primaries rather than metastases. Many of these patients also have coexisting endometriosis.
Clear Cell Tumors
Clear cell tumors are uncommon in the United States; they are more common in Japan. About half of cases are associated with endometriosis. Clear and “hobnail” cells are the microscopic hallmark, but the diagnosis rests upon architecture as well as optically clear cytoplasm. When present, the clear appearance of the cytoplasm results from glycogen that has leached as the tissue specimen is prepared for microscopic examination. The hobnail cells contain bulbous nuclei that protrude into the lumen at the apparent cytoplasmic limits of the cell.
Mixed Carcinoma
Perhaps, as many as 10% of all ovarian tumors of common epithelial origin are mixed, when defined as a carcinoma in which more than 10% of the neoplasm exhibits a second histologic cell type. One common specific malignant combination is mixed clear cell and endometrioid carcinoma, both being related to endometriosis.
Primary Peritoneal Serous Carcinomatosis
Primary peritoneal carcinoma has been defined by the Gynecologic Oncology Group (GOG) as follows: (i) ovaries are of normal size or enlarged by a benign process; (ii) ovarian involvement is absent or limited to the surface and/or superficial cortex with no tumor nodule within the ovarian cortex exceeding 5 × 5 mm; (iii) serous histology; and (iv) volume of extraovarian disease significantly exceeds that of ovarian disease. The pathology and response to treatment of peritoneal serous carcinomas are essentially identical to that of ovarian serous carcinomas. It has recently become apparent that the tubal fimbriae may be an important source of carcinomatosis in the absence of the typical features of primary tubal carcinomas (i.e., a dilated fallopian tube with a large mucosal lesion). Because the gross and microscopic characteristics, as well as spread, of carcinoma of the fallopian tube are identical to those of the ovary, the determination of the site of origin of a tumor that forms a solid or cystic tuboovarian mass is arbitrary. The fallopian tube may actually be the source of many high-grade serous carcinomas that are considered primary to the ovary.
Fallopian Tube Carcinoma
The gross appearance of the fallopian tube affected by papillary carcinoma is typically described as enlarged, deformed, or fusiform, with agglutination of the fimbriae and, frequently, distal obstruction. When the tumor is confined to the mucosa, the tube is generally soft to palpation, and the initial impression of the surgeon is often hematosalpinx, pyosalpinx, or hydrosalpinx. The most frequent site of origin is the ampulla, followed by the infundibulum. Microscopically, most fallopian tube neoplasms display papillary serous histology. With the recent knowledge that most high-grade serous carcinomas develop in the distal fallopian tube from precursor STIC lesions, the incidence of fallopian tube carcinoma has increased dramatically.
DIAGNOSIS AND CLINICAL EVALUATION
Ovarian carcinomas are distinctive by virtue of their propensity to exfoliate malignant cells into the peritoneal cavity. There the cells follow the normal circulation of peritoneal fluid up the right paracolic gutter and to the undersurface of the right hemidiaphragm, where they may implant and grow as surface nodules. The omentum is also a frequent site of involvement, and, indeed, all intraperitoneal (IP) surfaces are at risk. Ovarian cancer may also spread via the retroperitoneal lymphatics that drain the ovary. These follow the ovarian blood supply in the infundibulopelvic ligament to terminate in lymph nodes lying along the aorta and vena cava up to the level of the renal vessels. Lymph channels also pass laterally through the broad ligament and parametrial channels to terminate in the pelvic sidewall lymphatics, including the external iliac, obturator, and hypogastric chains. Spread may also occur along the course of the round ligament, resulting in involvement of the inguinal lymphatics.
Approximately 75% to 85% of patients with epithelial ovarian cancer are diagnosed at the time when their disease has spread throughout the peritoneal cavity. Several organizations, including the Gynecologic Cancer Foundation, the Society of Gynecologic Oncologists, and the American Cancer Society, released a consensus statement on the symptoms of ovarian cancer in June 2007. It urges women to seek medical attention if they have new and persistent symptoms of bloating, pelvic or abdominal pain, difficulty eating, early satiety, or urinary urgency or frequency. These symptoms were found to be much more likely to occur in women with ovarian cancer than in the general population. There is, however, no evidence that detection based on these factors will shift diagnosis early enough to affect mortality.
The diagnosis of early-stage ovarian cancer (when the tumor is still confined to the pelvis) usually occurs by palpation of an asymptomatic adnexal mass during a routine pelvic examination. However, the vast majority of palpable adnexal masses are not malignant and, in premenopausal women, ovarian cancer represents less than 5% of adnexal neoplasms. In these women, the ovarian enlargement is usually due to either follicular or corpus luteum cysts. The vast majority of these functional cysts will regress in one to three menstrual cycles and, consequently, the initial approach to management for a palpable adnexal mass less than 8 cm in size in a premenopausal woman is to repeat the pelvic examination and imaging studies in 1 to 2 months. In contrast, an adnexal mass in a premenarchal or postmenopausal woman, particularly when complex as seen on ultrasound, has a higher likelihood of being a malignant tumor, and surgical exploration is usually indicated.
Preoperative evaluation of the ovarian cancer patient is shown in Figure 10.3. CT scans of the chest, abdomen, and pelvis are usual. Brain scans and bone scans are unnecessary unless suggested by the patient’s symptoms; metastases to these sites are extremely uncommon, particularly at the time of diagnosis. If there is concern for a gastrointestinal primary based on bowel symptoms or heme-positive stools, barium enema or colonoscopy can be useful. Because of the association of ovarian cancer with breast cancer and because metastatic breast cancer can produce intra-abdominal carcinomatosis as well, mammography is often performed. Serum CA-125 level should be measured. It has been demonstrated that less than 1% of normal nonpregnant women have serum CA-125 levels greater than 35 U/mL. In contrast, 80% to 85% of patients with epithelial ovarian cancer have elevated serum levels. Unfortunately, the percentage of women with an elevated CA-125 level is lower in stage I disease, limiting the usefulness of the marker as a screening test. The serous histologic subset of ovarian cancer has the highest incidence of elevated CA-125 levels (>85%), whereas mucinous tumors are associated with a low incidence of abnormally elevated serum CA-125 levels. In postmenopausal women with asymptomatic pelvic masses, an elevated serum CA-125 (>65 U/mL) had a sensitivity of 97% and a specificity of 78% for ovarian cancer. In contrast, in premenopausal women, there is a higher prevalence of nonmalignant conditions that can produce elevated serum CA-125 levels (e.g., pregnancy, endometriosis, uterine fibroids, and pelvic inflammatory disease).
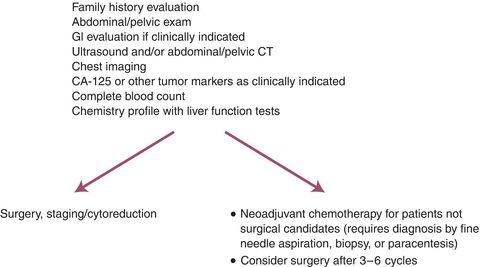
Figure 10.3 Suspicious symptoms/palpable mass or ascites.
SCREENING
KEY POINTS
- Ovarian cancer has a low prevalence: 1 case/2,500 women per year.
- Screening with pelvic exam, transvaginal ultrasound, and CA-125 has not been shown to be effective in any group of women, including BRCA mutation carriers.
- Premenopausal women frequently have palpable adnexal masses. Most are benign and are due to follicular or corpus luteum cysts.
- Surgical exploration is generally indicated for complex adnexal masses in postmenopausal women.
Despite decades of large trials, no reliable procedures are currently available for the early detection of ovarian cancer. Available potential screening techniques have included pelvic examination (ovarian palpation), ultrasound examinations, CA-125 and other tumor markers, and combined modality approaches. Difficulties include the need for surgery, with its attendant risks, to evaluate a positive test, and the fact that slow-growing tumors detected on screening are often borderline (which are not likely to be lethal) or already in an advanced stage (which are difficult to cure). A representative large multimodal screening trial, the NCI-sponsored Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, enrolled over 74,000 women aged 55 to 74 years from 1993 through 2000. Women were randomly assigned to either observation or baseline measurements of CA-125 levels and transvaginal ultrasonography followed by annual CA-125 readings for 6 years and transvaginal ultrasound for 4 years. Baseline screening in the 28,816 women randomized to screening who received at least one test detected 29 neoplasms (26 ovarian, 2 fallopian, and 1 primary peritoneal). Nine were of low malignant potential. Only 2 of the invasive cancers were stage I; 1 of these was a granulosa cell tumor. Five hundred seventy surgical procedures, including 325 laparotomies, were performed. The authors calculated the positive predictive value for invasive cancer of an abnormal CA-125 as 3.7%, of an abnormal transvaginal ultrasound as 1.0%, and of having both tests abnormal as 23.5%. However, if only subjects in whom both tests were abnormal had been evaluated, 12 of the 20 invasive cancers would have been missed.
Clearly, it is possible that screening may do more harm (complications from needless procedures) than good. At this time, routine screening of the general population should not be performed. Even in women with a positive family history of ovarian cancer or known BRCA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


