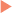CHAPTER 24 Outcomes of treatment for adult brachial plexus injuries
Summary box
Introduction
Management of brachial plexus (BP) injuries has changed significantly over the past century, especially with regard to our understanding of neurobiology, pathophysiology, and pathology of nerve injuries and regeneration, as well as the microsurgical repair of injured nerves.1,2,3,4,5,6 Advancements in the clinical diagnosis of nerve lesions, development of comprehensive and evidence-based treatment algorithms, and optimization of the intraoperative decision-making process resulting from the introduction of intraoperative nerve action potential (NAP) recordings paved the way for better management of patients inflicted with peripheral nerve and BP injuries.2,7 Another benefit of a rather standardized treatment approach is the ability to systematically study clinical outcomes of patients with nerve injuries after microsurgical repair. Multiple series of functional outcomes after nerve injuries have been reported, both in civilian populations and in war veterans.8,9,10 In this chapter, we discuss briefly our approaches in the management of patients with BP injuries, with particular emphasis on clinical outcomes after microsurgical repair.
Causes of surgical nerve injuries
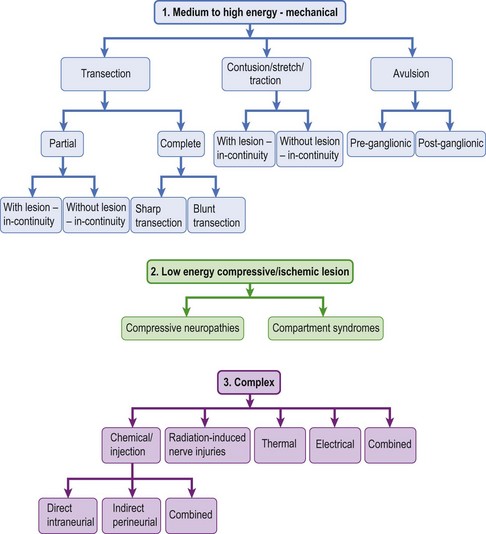
The BP can be injured either directly as in stab injuries where elements of the BP are sharply transected, or indirectly as in stretch injuries. Recently, traumatic nerve injuries have been classified based on the biomechanics of the injuring processes (Figure 24.1 (part 1)).11 This classification is different from the neuropathologic classification of nerve injuries by Sunderland (Table 24.1), as it emphasizes the mechanism of injury, which is important in deciding the best treatment option for a given nerve injury. BP injuries most often are caused by high energy forces that result in injuries such as transection, contusion, stretch, traction, and/or avulsion. However, BP injuries may also result from compressive neuropathies such as thoracic outlet syndrome, which are due to chronic or repetitive low energy forces (Figure 24.1 (part 2)). BP injuries from injections, radiation, and thermal energy involve rather heterogenous combinations of different injuring factors, but for the purposes of analysis, they are grouped together as a complex group of nerve injuries (Figure 24.1 (part 3)).11
Approach to clinical diagnosis
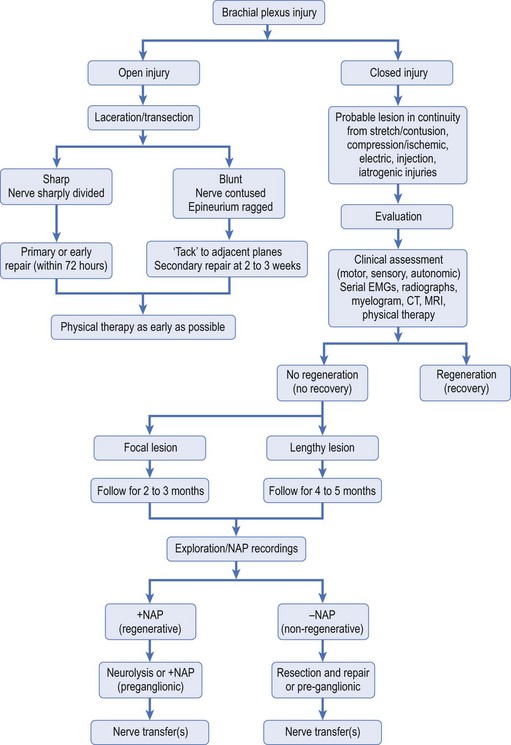
As with every other surgical discipline, the importance of detailed but relevant clinical patient history and mastery of physical examination of the patient as it relates to lesions of the BP cannot be overemphasized. Mastery of these skills combined with prudent use of ancillary tests, such as electromyography (EMG) and imaging studies, will guide the treating physician to an accurate diagnosis and localization of BP injuries (Table 24.2). It is of utmost importance to entertain a broad differential diagnosis and perhaps only accept the diagnosis of BP injury once other causes of upper extremity dysfunction have been considered. This approach is especially important when dealing with controversial diagnostic entities such as Parsonage-Turner and thoracic outlet syndrome. Once a diagnosis of BP injury has been established, surgical treatment of the lesion is dictated by nature of the injury (open versus closed), acuity of the injury (early versus delayed presentation), and findings on clinical, electrodiagnostic, and imaging studies (Figure 24.2).
Table 24.2 Role of nerve conduction studies and imaging in the diagnosis of nerve lesions
| With regard to EMG, the following should be emphasized: |
 Patients can have persistent paralysis despite nascents or reduction in denervational changes, such as fibrillation and denervation potentials. Patients can have persistent paralysis despite nascents or reduction in denervational changes, such as fibrillation and denervation potentials. Muscles related to neighboring nerves or plexus elements can show persistent EMG changes despite good function. Muscles related to neighboring nerves or plexus elements can show persistent EMG changes despite good function. |
| Imaging studies are important, but: |
 To date, in most institutions, MRI is not yet a substitute for CT-myelogram, especially when investigating root avulsions. To date, in most institutions, MRI is not yet a substitute for CT-myelogram, especially when investigating root avulsions. |
Indications and relative contraindications for surgery
Lacerations of the BP
Gunshot wound to BP
Indications for elective nerve repair of gunshot injuries to the BP are listed below:
Stretch Injuries to the BP
Management of stretch injuries to the BP has evolved significantly over the last century, beginning with predominantly non-surgical and musculoskeletal reconstructive operations and amputations to direct surgical repairs. Over the last 2 decades, our understanding of the pathophysiology of poor regeneration and functional recovery has improved significantly and, with it, the evolution of innovative approaches to microsurgical management of BP injuries.1,3,12,13 Likewise, better understanding of the natural history of BP injuries with regard to the potential for spontaneous recovery and timing of microsurgical repair has improved our capability to optimize patient benefit from surgical treatment. A combination of suboptimal clinical results despite excellent direct microsurgical repair, and our appreciation of proximo-distal discrepancy in the reinnervation of denervated muscles after BP injuries, created a receptive audience for Narakas when he re-popularized neurotization (ie, nerve transfers) procedures about 3 decades ago.14 We have adopted an approach to perform direct microsurgical repair for BP injuries whenever feasible, supplemented with neurotization procedures. Our indications for surgical exploration and repair of BP stretch injuries are as follows:
Approach to intraoperative management of BP lesions
Microsurgery
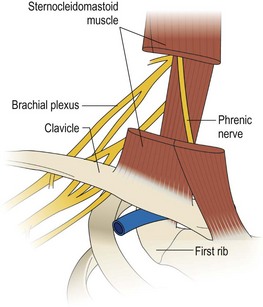
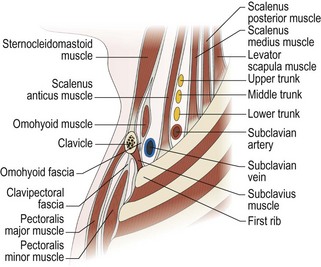
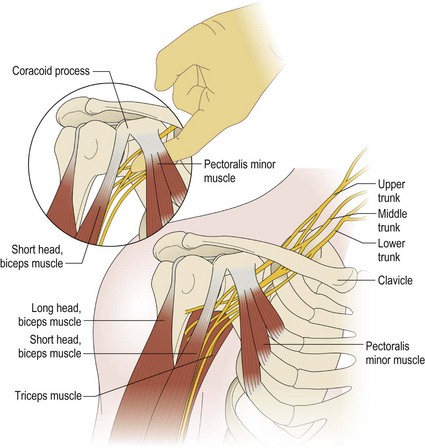
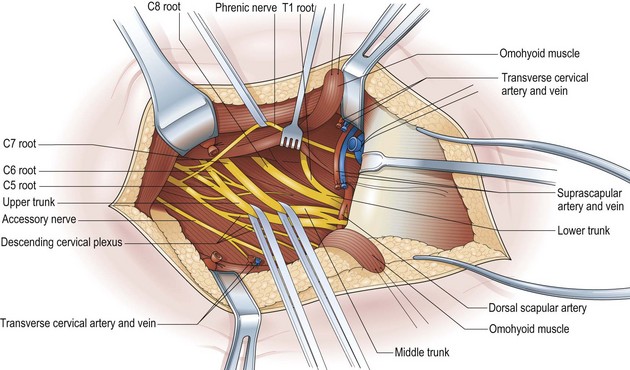
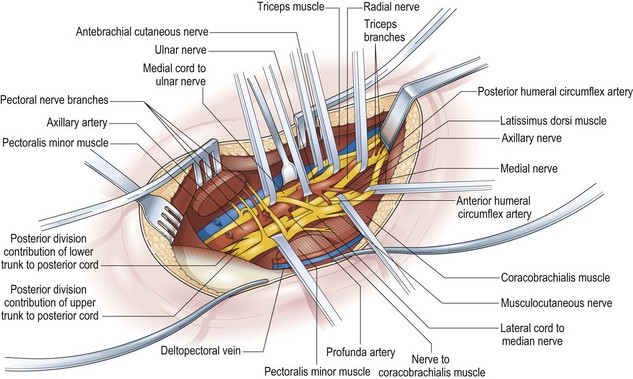
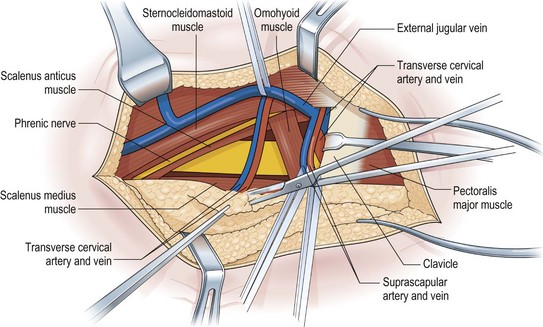
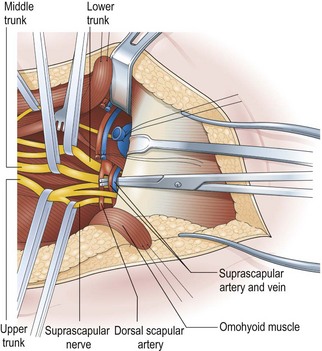
The key principles in nerve surgery include (1) thorough understanding of the topographic anatomy of the injured nerve (Figures 24.3, 24.4, 24.5); (2) mastery of the required incision and surgical approaches (Figures 24.6, 24.7); (3) adequate surgical exposure of the proximal and distal stumps of the injured nerves as well as the associated nerve branches and blood vessels (Figures 24.8, 24.9); (4) adequate magnification with the use of either the operating microscope or surgical loupes; (5) use of micro-instruments to minimize surgical trauma to the already traumatized nerves and/or branches; (6) careful application of bipolar coagulation only to the epineurial and intrafascicular bleeding points to minimize damage to the nerve itself.
Intraoperative nerve action potential (iNAP)
Lack of clinical and electrodiagnostic evidence of functional recovery 3 to 4 months after nerve injury are indications of lack of adequate reinnervation of denervated muscles and/or remyelination to generate muscle contractions. The senior author (DGK) pioneered the application of iNAP to the evaluation of axonal regeneration across a segment of damaged nerves.2,7 Intraoperative evaluation of axonal regeneration using iNAP is important, because it prevents inadvertent resection of nerves that still have the potential for spontaneous recovery. Appropriate intraoperative surgical decisions can be made with regard to the need for surgical resection of severely damaged nerves that have no response on iNAP (flat) and the subsequent need for direct or graft repair, or neurolysis, and continuation of observant non-surgical treatment if the damaged nerves have positive NAPs. Intraoperative NAPs can also help determine the proximal extent of healthy axons in injured nerves.
In addition, iNAP allows for early evaluation of nerve injuries and a determination of the extent of nerve damage. It can be applied 2 to 3 months after most focal injuries, and 3 to 4 months in less-focal lesions caused by stretch/contusion or gunshot injuries. With its application, the surgeon can sort out the management strategy for approximately 70% of nerve injuries in which nerve segments appear continuous with a variable amount of swelling and/or epineurial scar and yet are nonfunctional. NAP recordings can document the extent of a partial injury or prove that there is a neurapraxic block in the early weeks after the injury. When iNAP is applied months after injury, it can differentiate an axonotmetic lesion (positive NAP across the lesion) from a neurotmetic injury (negative NAP across the lesion).2,7
Nerve repair techniques and nerve grafts
The surgical objective of the repair of any severed peripheral nerve involves either microsurgical realignment of the injured nerve stumps (ie, primary neurorrhaphy) or the use of an autologous nerve graft to bridge a larger defect. A thorough understanding of the surgical anatomy of BP exposure at supraclavicular, clavicular, and infraclavicular levels allows the surgeon to expose key elements of the BP, even after stretch injuries where there is abundant scar tissue formation (Figures 24.10, 24.11, 24.12, 24.13, 24.14, 24.15). Wide exposure of BP elements and the application of iNAP guide the surgeon’s decision-making process with regard to microsurgical repair of injured nerves with or without nerve grafting. The nerve grafting technique was first reported between the years 1870 and 1900, but Hanno Millesi3 re-popularized the concept of using nerve grafts to bridge large defects to avoid the detrimental effects of suturing nerve stumps under tension.15 His work demonstrated that nerve grafting without tension was superior to epineurial suture under tension, and that tension at the repair site induces scar formation. Therefore, nerve repair without tension is most desirable, because the more scar tissue present at the repair site, the less satisfactory is functional recovery. Tension across a direct suture repair decreases blood flow and promotes proliferation of connective tissue within the nerve, which may block effective axonal regeneration.6,15 Acute, excessive stretch may cause intraneural hemorrhage resulting in scar formation and axoplasmic degeneration; subsequent maturation of scar tissue may shrink and constrict nerve fibers and may result in formation of a neuroma-in-continuity.6 However, whenever there are small-to-moderate gaps, it may be preferable to mobilize nerve ends to allow direct nerve repair (without tension), which results in better functional recovery than graft repair.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree