Objective
To conduct a systematic review and metaanalysis of studies evaluating the regression rate of endometrial hyperplasia with oral progestogens and levonorgestrel-releasing intrauterine system.
Study Design
Searches were conducted on Medline, Embase, Cochrane Library, and Web of Science, and reference lists of relevant articles were examined. The methodologic index for nonrandomized studies was used for quality assessment. Metaanalysis was performed with random effects model.
Results
There were 24 observational studies (1001 women), of low methodologic quality, evaluating the outcome of regression of endometrial hyperplasia with oral progestogens or levonorgestrel-releasing intrauterine system. Metaanalysis showed that oral progestogens achieved a lower pooled regression rate compared with levonorgestrel-releasing intrauterine system for complex (pooled rate, 66% vs 92%; P < .01) and atypical hyperplasia (pooled rate, 69% vs 90%; P = .03). There was no statistical difference in simple hyperplasia (pooled rate, 89% vs 96%; P = .41).
Conclusion
Oral progestogens appear to induce a lower disease regression rate than Levonorgestrel-releasing intrauterine system in the treatment of endometrial hyperplasia.
Endometrial hyperplasia is a common diagnosis (5-10%) in women presenting with abnormal uterine bleeding, and can progress to cancer if left untreated. The risk of progression of endometrial hyperplasia to cancer is dependent on the histologic diagnosis. The risk of cancer progression is low for women with complex nonatypical endometrial hyperplasia (3%) compared with women with cytologic atypia (8-29%). Nonsurgical therapeutic strategies in endometrial hyperplasia aim to induce disease regression and prevent progression to cancer. These strategies, if successful, could reduce the number of hysterectomies performed for this condition and hence reduce morbidity and health care costs.
Currently, there are no professional body guidelines for the management of endometrial hyperplasia. The use of progestogens, which antagonize the estrogen effect on the endometrium, can induce endometrial regression, and prevent progression to cancer. The main progestational agents used to treat endometrial hyperplasia are oral progestogens (norethisterone acetate, megestrol acetate, and medroxyprogesterone 17-acetate). More recently, the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena; Schering Health Care, Burgess Hill, UK) developed primarily as a contraceptive device, has also been used successfully to treat endometrial hyperplasia. This system has been proven to achieve higher concentrations of progestogens in the endometrium by almost 100-fold compared with oral administration. As it is not user dependent, compliance is 100%. We conducted a systematic review of studies evaluating oral and intrauterine progestogens for the treatment of endometrial hyperplasia and metaanalyzed their treatment effects.
Materials and Methods
Identification of literature
The population of interest in this systematic review was women with endometrial hyperplasia, the intervention was treatment with oral progestogens, the comparison was LNG-IUS, and the outcome was evidence of disease regression or persistence. The following electronic databases were searched: MEDLINE (1950 to December 2009), EMBASE (1980 to December 2009), Cochrane Central Register of Controlled Trials, and Web of Science conference proceedings (ISI Proceedings, 1990 to December 2009). A combination of Medical Subject Headings (MeSH) and text words were used to generate 2 subsets of citations, one including studies of endometrial hyperplasia (“endometr* hyperplas*”, “premalignant endometr*”, “precancer* endometr*”) and the other including studies of progestogens and intrauterine devices or systems (“intrauterine devices medicated”, “Levonorgestrel”, “mirena”, “intrauterine progest*”, “LNG-IU*”, “progest*”, “gestag*”). These subsets were combined with “AND” and limited to “Humans and Female” to generate a subset of citations relevant to our research question. The reference lists of all known primary and review articles were examined to identify cited articles not captured by electronic searches. Language or geographical restrictions were not applied during search or selection. The searches were conducted independently by I.D.G and T.K.P.
Study selection and data extraction
Studies were selected if the participants were women diagnosed histologically with endometrial hyperplasia, the intervention was treatment with either oral progestogens or LNG-IUS, and the outcome was histologic disease regression rates, as assessed on endometrial biopsy or hysterectomy specimen. Both controlled and uncontrolled designs were included. Case reports or series with <5 cases were excluded. Studies reporting on women with endometrial hyperplasia treated with other form of progestogens than oral or LNG-IUS (eg, injectable, pessaries) were excluded. Studies classifying women with endometrial hyperplasia in other than the World Health Classification of 1994 (simple, complex, and atypical) were also excluded.
Studies were selected in a 2-stage process. First, the titles and abstracts from the electronic searches were scrutinized by 2 reviewers independently (I.D.G. and M.S.) and full manuscripts of all citations that met the predefined selection criteria were obtained. Secondly, final inclusion or exclusion decisions were made on examination of the full manuscripts. In cases of duplicates, the most recent or the most complete publication was used. Any disagreements about inclusion were resolved by consensus or arbitration by a third reviewer (A.C.).
Two reviewers (I.D.G. and M.S.) completed the quality assessment. The Methodologic Index for NonRandomized Studies (MINORS), which assesses the quality of the included studies, was implemented. Items assessed included selection of cases or cohorts and controls, comparability and information on exposure and outcome. This index was preferred over Newcastle-Ottawa Quality Assessment Scale as we included studies without a control group and the MINORS checklist allows a quality evaluation in studies with and without a control group. From each study, outcome data were extracted in 2×2 tables by the 2 reviewers I.D.G. and M.S. No ethical approval was sought for this study as it was a systematic review and metaanalysis of published articles.
Statistical analysis
Regression rates from individual studies were metaanalyzed using a random effects model. Heterogeneity of the exposure effects was statistically analysed using the χ 2 test. Exploration of the causes of heterogeneity was planned using variation in features of population, exposure, and study quality. The regression rates between the 2 interventions (oral progestogens and LNG-IUS) were compared with the aid of metaregression. Statistical analyses were performed using Stata 8.0 (Stata Corp, College Station, TX).
Results
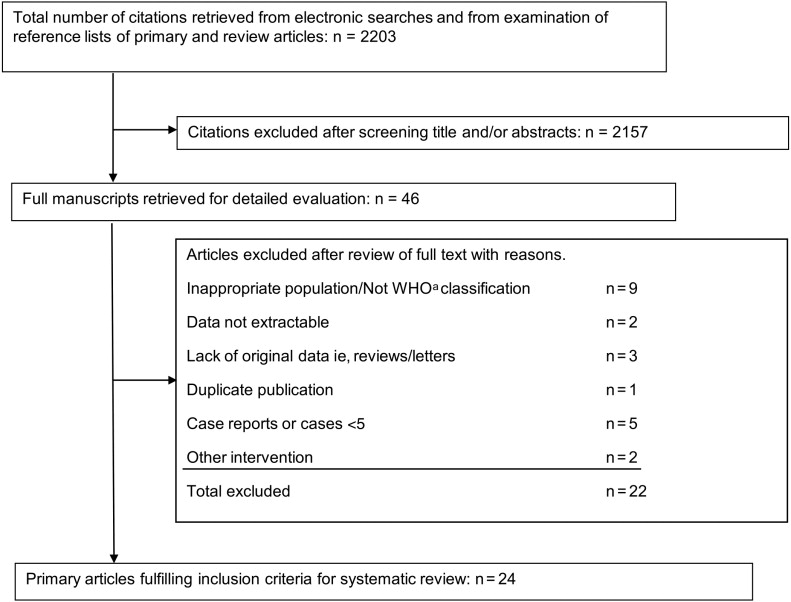
The search strategy yielded 2203 citations all captured from electronic citations. Of these, 2157 were excluded as it was clear from the title and abstract that they did not fulfill the selection criteria. Examination of the full manuscripts of the remaining 46 articles found that 3 studies lacked original data (eg, reviews or letters), 1 study was a duplicate, and 18 studies did not meet the selection criteria. Thus, a total of 24 primary studies, including 1001 women with endometrial hyperplasia were selected for this review ( Figure 1 ). The longest follow-up period was 8 years. Fifteen studies were case series and 9 were controlled studies. The main characteristics of the 24 studies and the MINORS Index are presented in Figure 2 and the Table . Although all studies included women with either oral progestogens or LNG-IUS, the type, dose, regimen, and duration of treatment varied. The type of hyperplasia (simple, complex, or atypical) treated also varied between the different studies. Most studies were judged to be of poor quality on the MINORS index ( Figure 2 ), with particular low scores for prospective calculation of the study size, prospective recruitment, and biased assessment of regression rates.