19 Ophthalmology
Anatomy of the Visual System
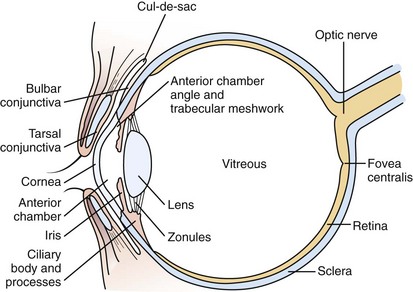
The visual system is conveniently separated into three principal parts: the globe and surrounding structures (Fig. 19-1), the visual pathways, and the visual or calcarine cortex.
The eyelids provide protection for the globe and assist in even distribution of the tear film over the cornea to provide a clear, undistorted optical surface. The crystalline lens complements the cornea’s refracting power with its ability to adjust the focal length of the optical system so that incoming light from objects at any distance may be clearly imaged on the retina. During the first 2 to 3 months of life, children develop the ability to focus on images at any range (accommodation). Light is focused on the macula, the portion of the retina responsible for the central field of vision (Fig. 19-2). The retina contains the photoreceptors, rods and cones, which convert light energy into an electrical nerve response. The fovea centralis is the center of the macula; it has the greatest concentration of cones and is responsible for detailed, colored central visual acuity.
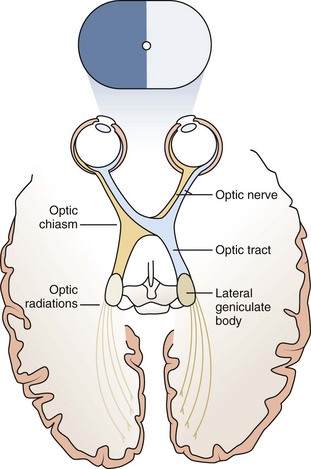
Nerve fibers emanate from the ganglion cell layer of the retina and coalesce to form the optic nerve. The right and left optic nerves pass from the orbits and join together to form the optic chiasm. Nerve fibers from the nasal retina decussate at the chiasm and are directed toward the contralateral side of the brain. Fibers from the temporal retina travel without crossing at the chiasm to the ipsilateral visual cortex (Fig. 19-3). From the optic chiasm the fibers form the optic tracts and synapse in the lateral geniculate nucleus in the midbrain. From there the optic radiations, one on each side of the brain, travel posteriorly to the visual cortex above the cerebellum.
Evaluation of Vision
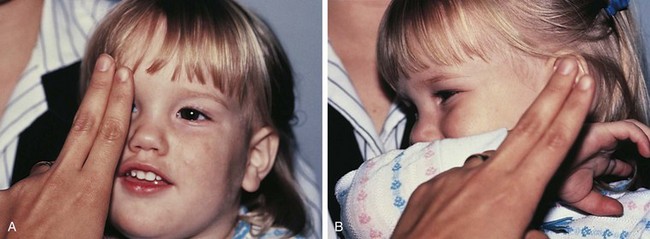
Selection of a test to measure a patient’s visual acuity depends on the patient’s age, cooperation, and level of development. The evaluation of vision in a young infant or nonverbal patient requires the use of the fixation reflex. This reflex develops during the first month or two of life. Although almost all 2- to 3-month-old infants can fixate and follow a face or bright object quite well into all fields of gaze, it is not necessarily abnormal for this milestone not to be present until 6 months of age. The level of vision can be estimated by the quality and intensity of the fixation response. If the visual acuity is normal, central fixation is steady and maintained on objects. If visual acuity is profoundly decreased, the quality of fixation may be wandering in nature, poorly maintained, or eccentric. Central, steady, maintained fixation equates to visual acuity of 20/200 or better. Eyes with unsteady or wandering fixation usually have visual acuity decreased to the 20/800 range (Fig. 19-4).
The Allen object recognition cards, simple pictures of common objects, are useful for assessing visual acuity in a 2- to 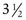 -year-old child who cannot comprehend the illiterate or tumbling E game or recognize Snellen letters. To perform this test, the child is taught what the pictures are, and then one eye is occluded and picture cards are individually presented at increasing distances until the patient recognizes the cards at 20 feet or fails to recognize the cards (Fig. 19-5). Recognition of the cards at a distance of 20 feet equates to a visual acuity of 20/30. The farthest distance at which the cards can be recognized is noted, and the visual acuity is quantitated as that distance over the denominator of 30 (e.g., 5/30, 15/30, 20/30). This is a measurement of recognition visual acuity. Although use of isolated targets is not ideal for detection of amblyopia, the test can be quickly and easily taught to apprehensive or shy young children and comparison of vision between the eyes detects most cases of amblyopia and other defects in visual acuity.
-year-old child who cannot comprehend the illiterate or tumbling E game or recognize Snellen letters. To perform this test, the child is taught what the pictures are, and then one eye is occluded and picture cards are individually presented at increasing distances until the patient recognizes the cards at 20 feet or fails to recognize the cards (Fig. 19-5). Recognition of the cards at a distance of 20 feet equates to a visual acuity of 20/30. The farthest distance at which the cards can be recognized is noted, and the visual acuity is quantitated as that distance over the denominator of 30 (e.g., 5/30, 15/30, 20/30). This is a measurement of recognition visual acuity. Although use of isolated targets is not ideal for detection of amblyopia, the test can be quickly and easily taught to apprehensive or shy young children and comparison of vision between the eyes detects most cases of amblyopia and other defects in visual acuity.
The Sheridan-Gardiner or HOTV visual acuity tests are easy to administer and more accurately measure visual acuity in 4- to 6-year-old children who are beginning to read letters. In the HOTV test the letters H, O, T, and V are individually presented on cards, and the child matches the letter with a corresponding letter on a card that is held on the lap (Fig. 19-6).
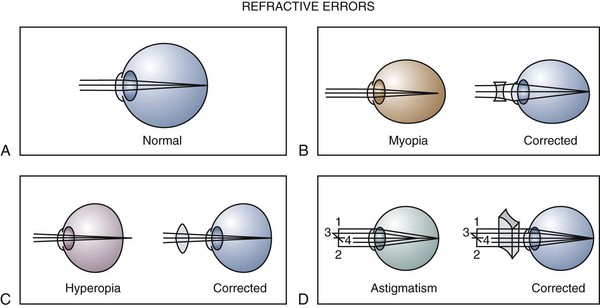
Refractive Errors
Decreased visual acuity is most commonly the result of a refractive error in the eye. This may be due to variation in the curvature of the cornea or lens or variation in the axial length of the eye. Determination of the refractive state of the eye is part of a comprehensive ophthalmic evaluation. In children, an objective measurement of the refractive error is best obtained by using eyedrops that temporarily inhibit accommodation (cycloplegia) and cause pupillary dilation (mydriasis). Cycloplegic–mydriatic agents such as cyclopentolate or tropicamide are instilled, and 30 minutes later accommodation is temporarily paralyzed and the pupil is dilated. A retinoscope is used to project a beam of light into the eye and illuminate the retina. The light is then reflected back to the examiner through the patient’s pupil and optical system. The focus of the reflected light is neutralized by placement of appropriate lenses in front of the eye, and the refractive error of the eye is accurately and objectively measured (Fig. 19-7).
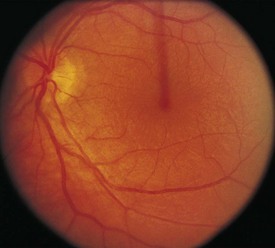
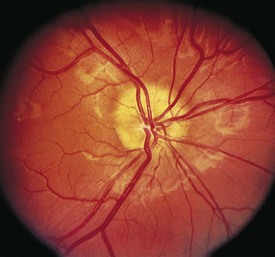
The optical image formed by a hyperopic eye is in focus behind the retina (Fig. 19-8, C). By changing the shape of the lens with accommodation, the image can be brought into focus on the retina and glasses may not be required. If a large amount of hyperopia is present (+4.00 diopters or more), fatigue; headaches; asthenopia; and blurring of vision, especially at near, may occur. Hyperopia greater than +5.00 or +6.00 diopters may cause ametropic amblyopia. When this occurs, glasses are prescribed to correct the child’s refractive error so that focused images stimulate the development of normal vision. If large hyperopic refractive errors are not treated by 6 to 8 years of age, the resultant amblyopia may be irreversible. The optic discs in eyes with large degrees of hyperopia may have an appearance simulating papilledema (pseudopapilledema) (Fig. 19-9).
Myopia is most commonly caused by an increase in axial length of the eye with respect to the optical power of the eye (see Fig. 19-8, B). Children who are myopic can see near objects clearly; objects at distance are blurred and cannot be brought into focus without the aid of glasses or a contact lens. Wearing glasses for myopia does not change the growth of the eye and, contrary to popular belief, does not promote the resolution or progression of the myopia. Bifocals and cycloplegics may have a small effect on slowing the progression of myopia, but no treatment is currently available to reverse or stop the progression of myopia.
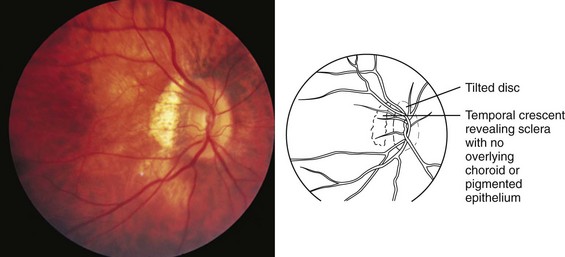
High degrees of myopia ranging from −8.00 to −20.00 diopters may be associated with systemic conditions such as Stickler and Ehlers-Danlos syndromes, which are associated with connective tissue defects and increased axial length of the eye. Myopia is inherited as a multifactorial trait. High myopia with extreme lengthening of the globe may be associated with retinal thinning, peripapillary pigment crescents, staphylomas (a focal area of bulging of the posterior globe wall), and decreased macular function with poor visual acuity. The optic nerve may appear to enter the eye at an angle (Fig. 19-10). High myopia has an increased incidence of retinal detachment, especially after direct trauma to the eye or concussive head trauma.
In astigmatism the refractive power of the eye is different in different meridians (see Fig. 19-8, D). This produces a blurred retinal image for objects at both distance and near. Astigmatism occurs when the cornea, lens, or retinal surface has a toric shape rather than a spherical one. This may be likened to the two different radii of curvature that give a football its characteristic shape. Bulky masses in the lids such as chalazions or hemangiomas may compress the cornea and induce astigmatic refractive errors.
Strabismus
The third, fourth, and sixth cranial nerve nuclei, located in the brainstem, are the centers responsible for innervating the extraocular muscles. In addition to innervation of the inferior oblique, medial, inferior, and superior recti, the third cranial nerve is responsible for innervation of the levator muscle, pupillary constriction, and accommodation of the lens. The fourth cranial nerve provides innervation to the superior oblique muscle, and the sixth cranial nerve supplies the lateral rectus muscle (Fig. 19-11). When one or more of the cranial nerves are paretic, the action of the innervated muscle is decreased, leading to a deficit in the duction or movement of the eye into the field of action of the muscle. The muscle having a function of movement in the opposite direction is no longer balanced, producing strabismus.
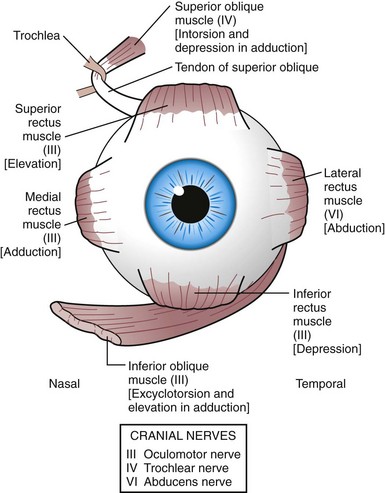
Figure 19-11 Innervation (in parentheses) of extraocular muscles and their primary actions [in brackets].
Esodeviations
Infantile Esotropia
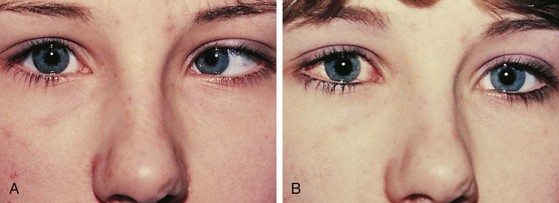
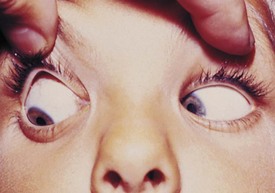
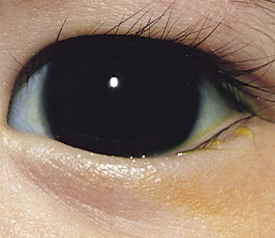
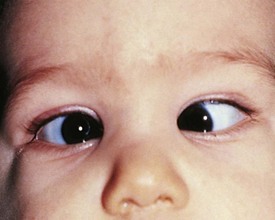
The most common cause for an esodeviation presenting in infancy is infantile, or congenital, esotropia (Fig. 19-12). In this condition the esotropia is seen at birth or very early in infancy. On occasion a family history of infantile esotropia exists. The angle of esodeviation is large and constant. Abduction may be deficient due to contraction of the medial rectus muscles, and differentiation from sixth nerve palsy may be difficult. Cross-fixation is usually present, with the adducted right eye used for vision to the left and the adducted left eye used for vision to the right. Abduction of an eye is checked by occluding the contralateral eye and quickly encouraging fixation movements while holding the head. Doll’s head maneuvers may also be used to test abduction of the eyes. Children with this condition usually do not develop much amblyopia and maintain good visual acuity in both eyes whether they are treated or not. There are no associated neurologic or systemic abnormalities.
The esodeviation present in infantile esotropia requires surgical correction. After correction, the ocular alignment is frequently unstable, with further surgery commonly required later in life no matter how early surgery is performed or how well aligned the eyes are after surgery. Inferior oblique overaction and dissociated vertical deviations frequently develop later in childhood or adolescence. Inferior oblique overaction is seen as an elevation of one or both eyes in adduction. Dissociated vertical deviation (DVD) (Fig. 19-13) is an upward and outward “floating” movement of one or both eyes that becomes more prominent with fatigue or inattention. A DVD may be elicited on examination by covering one eye and watching its position as the eye is covered. These patients do not experience diplopia. Patients with infantile esotropia also commonly develop accommodative esotropia, with a need for glasses later in childhood. While excellent visual acuity and alignment of the eyes are most commonly achieved with treatment, the development of high levels of binocular function (stereopsis) is usually not obtained.
Accommodative Esotropia
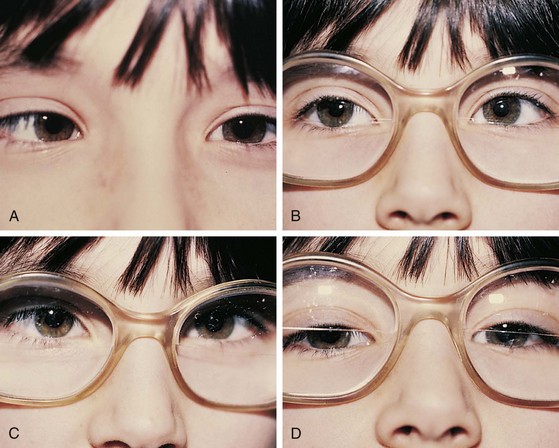
If an esodeviation is associated with a modest degree of farsightedness, treatment of the hyperopia optically with glasses is indicated. In patients with purely accommodative esotropia, this measure alone may completely correct the deviation (Fig. 19-14). Frequently, especially if the esodeviation has been left untreated for a long period of time, a residual esodeviation will remain. This is a partially accommodative esotropia. Surgical correction may be recommended for these patients. Some patients have straight eyes for some time with their glasses in place and decompensate to partially accommodative or nonaccommodative esodeviations later.
In another form of accommodative esotropia, the ratio between accommodative convergence and accommodation (AC/A ratio) may be abnormally high, producing excessive convergence when focusing on near objects. In high AC/A ratio accommodative esotropia, the esodeviation with near vision is greater in magnitude than it is with distance vision (Fig. 19-15). These patients may be treated with a bifocal, which gives them additional hyperopic correction for near, decreasing their accommodative effort at near and decreasing the near esodeviation.
Nonaccommodative Esotropia
Children may develop an esodeviation that is present without any relationship to the patient’s refractive error (Fig. 19-16). Correction of the hyperopia with glasses does not improve the esotropia in these patients. These nonaccommodative esodeviations may be associated with poor vision, trauma, prematurity, aphakia, or high myopia. Nonaccommodative esotropia may also develop when accommodative esotropias are left untreated.
Other Causes of Esotropia
Duane syndrome is a congenital unilateral or bilateral defect characterized by inability to abduct an eye. This may be accompanied by an up or down shoot of the eye and narrowing of the lid fissure on attempted adduction. In attempted abduction the palpebral fissure widens. Duane syndrome is caused by a malformation of the cranial nerve nuclei producing co-innervation of the medial and lateral rectus muscles. The lateral rectus muscle does not contract with abduction and paradoxically co-contracts along with the medial rectus on adduction. The co-contraction with adduction causes a retraction of the globe and the lid fissure narrowing. Patients with Duane syndrome may be esotropic and have a head turn toward the involved side analogous to those seen with a sixth cranial nerve palsy. The changes in lid position and vertical deviations help to differentiate the two conditions (Fig. 19-17).
Pseudostrabismus
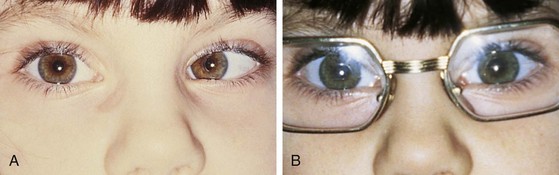
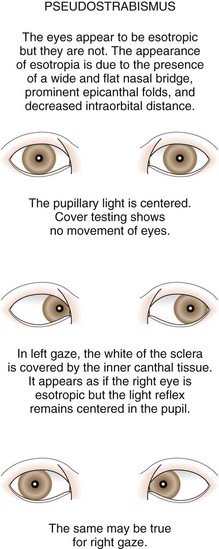
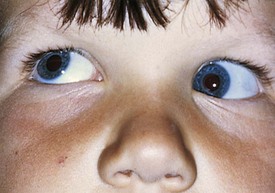
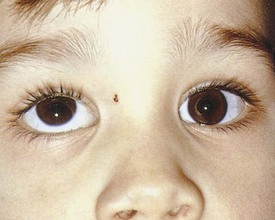
Pseudostrabismus is seen in infants with prominent epicanthal folds, closely placed eyes, and flat nasal bridges. Asymmetry of the lids or nasal bridge may also produce pseudostrabismus. When these facial features are present, the white of the sclera between the cornea and inner canthus frequently may be obscured, giving the optical illusion that the eyes are esotropic (Figs. 19-18 and 19-19). Parents and caretakers frequently report subtle esodeviations that worsen with gaze to the right or left. This is frequently pointed out in photographs in which careful examination shows the eyes to be in slight right or left gaze. Observation of symmetrical corneal light reflexes or cover testing confirms or excludes the presence of a true deviation.
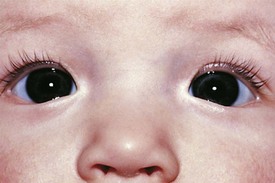
Figure 19-18 This infant has pseudostrabismus, caused by a flat nasal bridge, epicanthal folds, and closely placed eyes.
Exodeviations
When the visual axes are divergent, an exodeviation is present (Fig. 19-20). Many children with exodeviations have family histories of strabismus. Exodeviations may also occur with vision loss in one eye (sensory exotropia) and cranial nerve paralysis (third nerve palsy). An exodeviation may be controlled by fusion (exophoria), be manifest intermittently (intermittent exotropia), or be constant (exotropia).
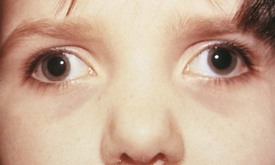
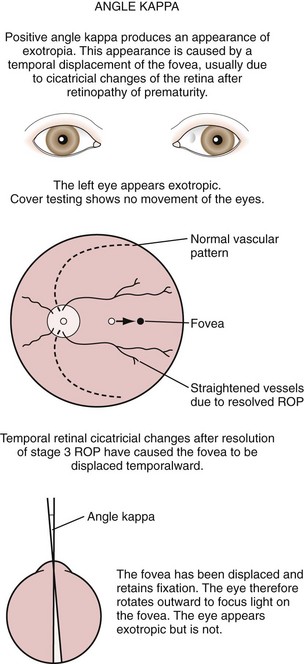
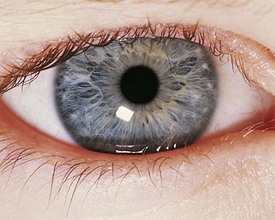
Exodeviations may be simulated in patients with widely spaced eyes (hypertelorism) or in those whose maculae are temporally displaced, as may occur in retinopathy of prematurity. When the macula is displaced temporally, the eye rotates outward to align its visual axis on the fixation target. The term positive angle kappa is used to describe this condition (Fig. 19-21).
Third (Oculomotor) Cranial Nerve Palsy
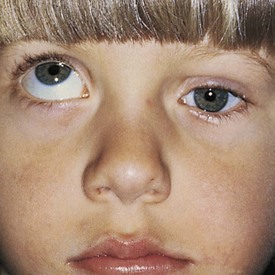
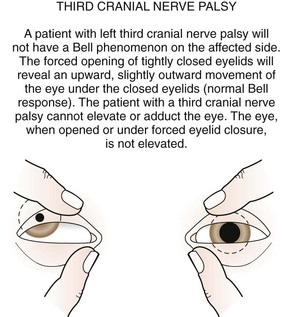
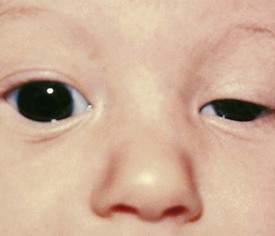
The third cranial nerve innervates the medial rectus muscle. In third nerve paralysis the action of the lateral rectus muscle, innervated by the sixth cranial nerve, is unopposed and produces an exodeviation. The third nerve also innervates the superior and inferior recti; the inferior oblique muscles; the levator palpebrae superioris, which elevates the lid; the ciliary muscle, which is responsible for accommodation of the lens; and the iris sphincter muscle, which produces miosis of the pupil. In the presence of a complete third cranial nerve palsy, the eye assumes a down and outward position, the eyelid is ptotic, and the pupil is enlarged (Fig. 19-22). Elevation of the eye with forced eyelid closure (the Bell phenomenon) is typically absent in patients with a third cranial nerve palsy (Fig. 19-23). The most common causes for acquired third nerve paralysis in children are trauma and tumor. A third nerve palsy may also occur as a congenital defect.
Vertical Deviations
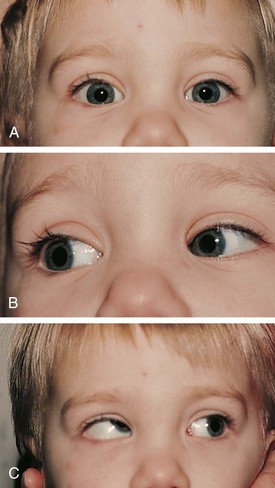
The most common cyclovertical deviation is due to a palsy of the fourth cranial (trochlear) nerve (Fig. 19-24). Fourth nerve palsies in children occur congenitally or secondary to head trauma. The eye is excyclorotated, and the head is tilted to the shoulder opposite the side of the paretic nerve and superior oblique muscle. Other features are elevation of the eye and difficulty depressing the eye in adduction (Fig. 19-25). Patients with fourth nerve palsies have diplopia in the contralateral field of gaze, especially up and away from the paretic side. Patients with congenital palsies may not always recognize this diplopia. Some facial asymmetry, especially of the cheek and jaw line, is usually seen in congenital cases as the children age. Examination of candid photos will typically display head postures that usually are not noticed by family members.
Brown syndrome describes an isolated motility disorder in which there is an inability to elevate the eye when it is adducted (Fig. 19-26). This may be caused by a congenital anomaly of the superior oblique tendon, or it may be acquired as an idiopathic inflammation or tenosynovitis of the superior oblique tendon. Acquired cases may be persistent, resolve spontaneously (sometimes over many years), or respond to nonsteroidal antiinflammatory drugs.
Tests for Strabismus
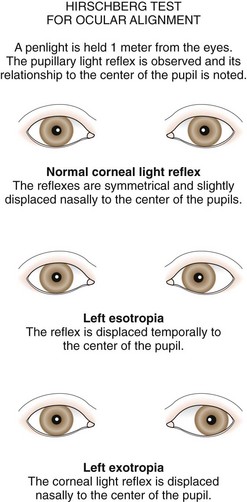
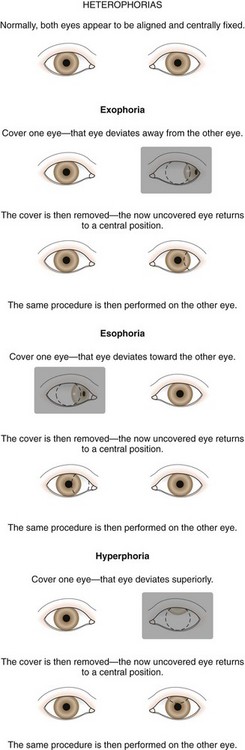
The type and degree of ocular misalignment may be estimated using the corneal light reflex test, or Hirschberg method. The patient fixates on a penlight held at 1 m. Using the pupil edge as a point of reference, the light reflections between the two eyes are compared for symmetry; if the light reflex is displaced temporally in one eye compared with the reflex seen in the other eye, an esotropia is present. If the light reflex is displaced nasally in comparison with the other eye, an exodeviation is present (Fig. 19-27). Although observation of the corneal light reflexes is more sensitive and specific than gross observation alone, a more accurate method of detecting misalignment of the eyes is cover and uncover testing.
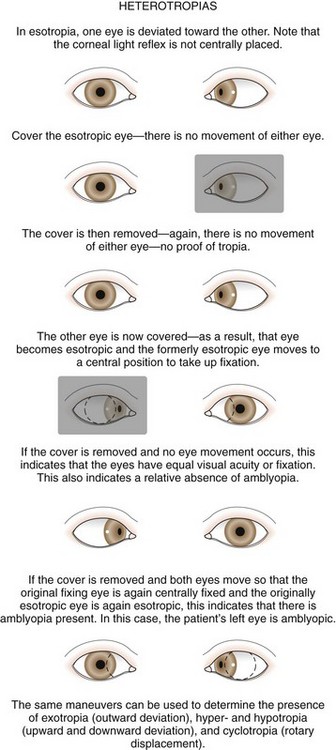
The cover test requires vision in each eye and use of a target that stimulates accommodation. Cover testing is performed while the patient maintains fixation on targets at 6 m and at 1 m because some types of strabismus produce misalignment of the eyes that is present only at either distance or near. The cover–uncover test is used to detect phorias. This test is performed by placing a cover over one eye to disrupt fusion or binocularity. As the cover is removed, the previously covered eye is observed. If the eye does not move, both eyes are aligned on the object at that distance; orthophoria is present. If the eye deviates while covered and then moves to regain fusion and assumes fixation as the cover is removed, a phoria exists. The test is then repeated, covering and uncovering the other eye (Fig. 19-28).
The second component of the cover test is performed by covering one eye and observing the movement of the other eye. If neither eye moves as the eyes are alternately covered, the eyes are both aligned on the fixation target and the term orthophoria is used. No deviation is present in this case. If a tropia and a fixation preference are present, a fixation movement of the deviating uncovered eye occurs when the preferred fixating eye is covered; when the cover is transferred back, the previously deviating eye again deviates behind the cover (Fig. 19-29). If a deviation is well controlled by fusion (a phoria) and is small in size, it may be safely observed if there are no symptoms and the fundus is normal. When a tropia is present, either constantly or intermittently, after 3 months of age, the patient should be referred to an ophthalmologist. Ophthalmologists use prisms along with cover testing to measure the size of strabismic deviations.
Amblyopia
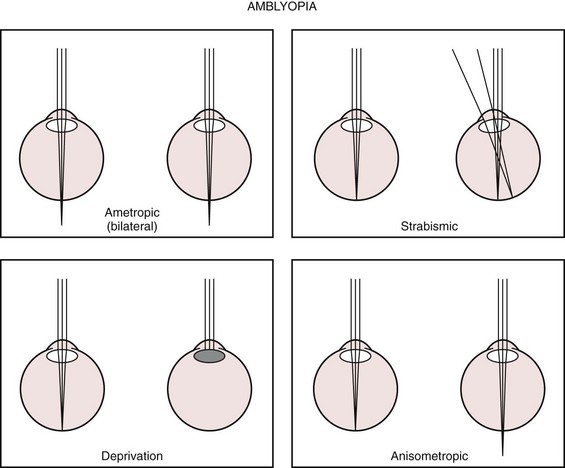
Amblyopia is present when there is a decrease in vision in one or both eyes and all potential organic causes (refractive errors, media opacities, structural abnormalities) for the decrease in vision have been corrected or excluded. Amblyopia may be caused by the absence of stimulation of the immature visual system by a focused retinal image or by strabismus and the resultant suppression of one eye. Visual deprivation amblyopia may be caused by a corneal opacity, a dense cataract, vitreous opacity (hemorrhage or inflammation), or high refractive error (Fig. 19-30).
Diseases of the Eyes and Surrounding Structures
Eyelids and Adnexa—Anatomy of the Eyelid
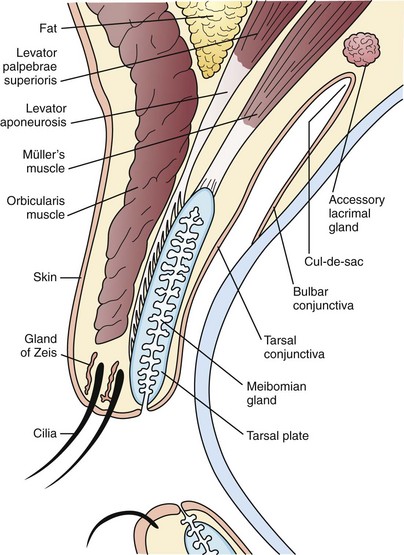
The eyelid is composed of skin and its related appendages, glands that contribute to the tear film, and muscular structures permitting the eyelid to open and close (Fig. 19-31). Conditions affecting the eyelid are related to these anatomic structures.
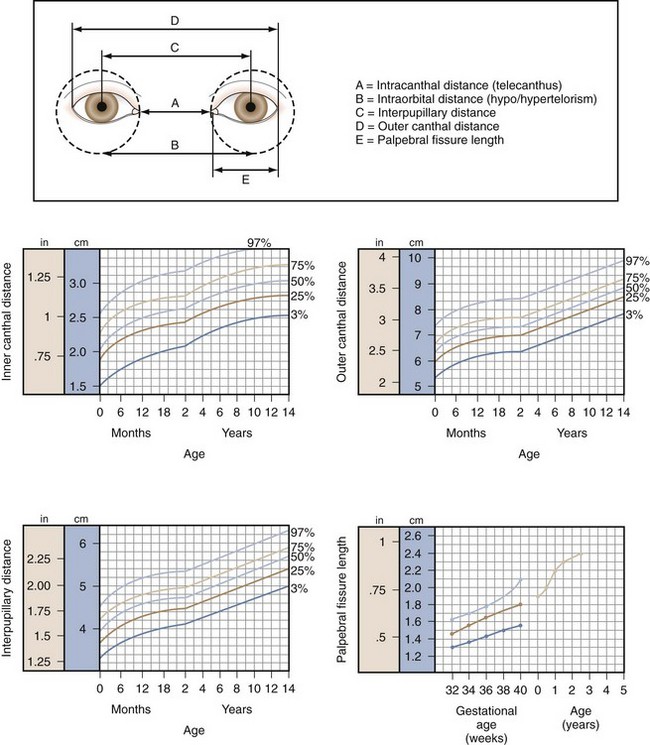
Telecanthus refers to an increase in the distance between the inner canthus of each eye (Fig. 19-32). Telecanthus can be due to the hereditary transmission of facial features or midline embryonic defects, or it can be related to a syndrome such as blepharophimosis, or Komoto syndrome (Fig. 19-33). This inherited syndrome consists of telecanthus, epicanthus inversus (a skinfold projecting over the inner angle of the eye and covering part of the canthus, arising from the lower lid skin), blepharophimosis (horizontal shortening of the lid fissure), and ptosis. Hypertelorism refers to an increase in the distance between the nasal walls of the orbits. This is usually associated with telecanthus.
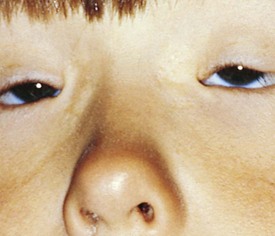
Figure 19-33 Komoto syndrome, a combination of blepharophimosis, ptosis, epicanthus inversus, and telecanthus.
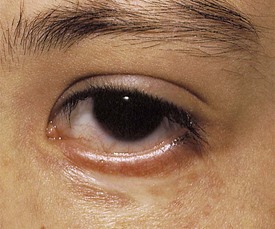
Blepharoptosis, or ptosis, is a unilateral or bilateral decrease in the vertical distance between the upper and lower eyelids (palpebral fissure) because of dysfunction of the levator muscle (Fig. 19-34). Congenital blepharoptosis is frequently transmitted as an autosomal dominant trait with variable penetrance. Congenital ptosis may be either unilateral or bilateral. Other causes for blepharoptosis include ocular inflammation, chronic irritation of the anterior segment of the eye, chronic use of topical steroid eye drops, third nerve palsy, and trauma. Ptosis may be severe enough to cause visual deprivation and amblyopia if the visual axis is occluded. Children with even mild degrees of congenital ptosis should be referred for evaluation because they have a higher than normal incidence of strabismus and anisometropic amblyopia than the general population. In congenital ptosis the eyelid position may improve somewhat from that observed early in infancy, but after that it tends to remain stable or worsen only slightly over time.
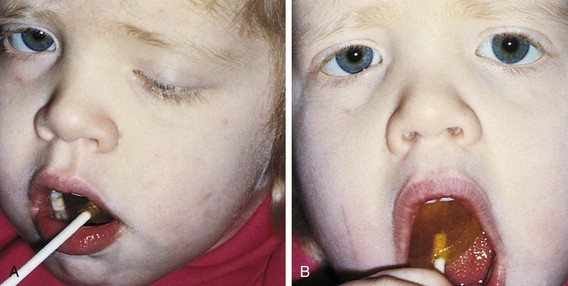
The Marcus Gunn, or jaw-winking, phenomenon is caused by a misdirection of the motor division of the fifth cranial nerve to the ipsilateral levator muscle of the eyelid (Fig. 19-35). With jaw movement to the ipsilateral side the eyelid droops, and when the jaw is moved to the contralateral side, the eyelid elevates. The eyelid winks with chewing or feeding. This is a benign phenomenon and no further neurologic or systemic evaluation is required. Surgical treatment is not indicated unless the associated ptosis warrants it. Most patients learn to control the winking by avoiding the inciting jaw movement.
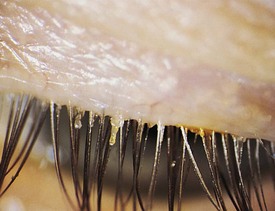
Districhiasis describes a condition in which there is an accessory row of eyelashes (cilia) along the posterior border of the eyelid (Fig. 19-36). Eyelid eversion or ectropion frequently coexists because of defects in the tarsal plate. This condition is inherited as an autosomal dominant condition, but it may also be a sequela of severe ocular inflammation.
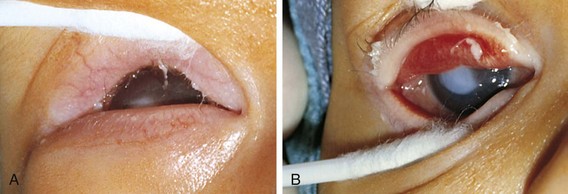
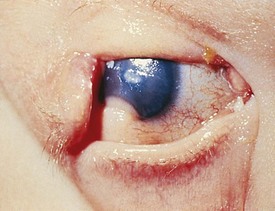
Entropion is an inverted eyelid with the lashes rubbing against the conjunctiva or cornea. This may be present at birth or occur with severe blepharospasm, inflammation, or trauma. If severe, abrasion of the cornea by the lashes can cause permanent corneal scarring (Fig. 19-37).
In epiblepharon a skinfold extends over the lid margin and presses the lashes against the globe. It is commonly observed during the first year of life (Fig. 19-38). The lower lid is more commonly affected in the white population, and both the upper and lower lids may be affected in Asian infants. This defect usually corrects itself spontaneously by 1 year of age. In Asians the problem may be persistent. Corneal abrasion usually does not occur because of the soft texture of the infant’s eyelashes or when it is the shaft of the eyelash rather than the tip of the lash that touches the cornea. However, surgical correction may be required if it is persistent and causing corneal abrasion; conjunctival injection, epiphora, and photosensitivity are symptoms in more significant cases.
Ectropion is an outward rotation of the eyelid margin. If severe, ectropion can lead to problems of corneal exposure. Ectropion may be congenital or caused by any condition (trauma, scleroderma), causing the eyelid skin to contract and evert the eyelid (Fig. 19-39). Ectropion may occur after seventh cranial nerve palsy with paralysis of the facial musculature.
Congenital eyelid colobomas are defects or notches in the eyelid margin caused by failed fusion of embryonic fissures early in development. These may be isolated defects or associated with conditions such as Goldenhar syndrome (Fig. 19-40). Goldenhar syndrome consists of eyelid colobomas, corneal–limbal dermoids, vertebral anomalies, and preauricular skin tags.
Children may frequently have a low-grade inflammation of the eyelid margin, chronic blepharitis, caused by Staphylococcus infection of the oil glands of the lid margin. Blepharitis may be associated with seborrhea or allergies and occurs commonly in children with Down syndrome. Symptoms include crusting of the lashes, itching, light sensitivity, and irritation of the lids. The lashes may be matted and adherent in the morning. If the condition is chronic and severe, thickening of the eyelid and misdirection of the eyelashes to such a point that they may invert and irritate the cornea or conjunctiva may occur (Fig. 19-41). Complications include ulceration of the lid margin, abscess or hordeolum formation, chronic conjunctivitis, and keratitis (corneal irritation and inflammation).
A hordeolum is an inflamed gland of Zeis at the base of the cilia (Fig. 19-42). This produces painful swelling and erythema of the eyelid. These lesions are commonly called styes. Infection, frequently with Staphylococcus, may occur. Some discharge may be seen. Rarely, preseptal cellulitis may occur as a complication.
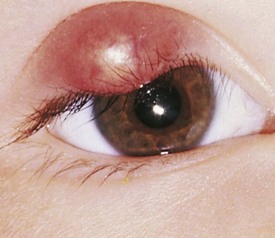
Figure 19-42 Acute hordeolum of the eyelid (pointing externally) with swelling, induration, and purulent contents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




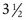 -year-old children is the “E game.” The child is presented with the letter E in decreasing sizes and rotated in an up, down, right, or left orientation, and the child indicates the direction of the crossbars of the E by pointing.
-year-old children is the “E game.” The child is presented with the letter E in decreasing sizes and rotated in an up, down, right, or left orientation, and the child indicates the direction of the crossbars of the E by pointing.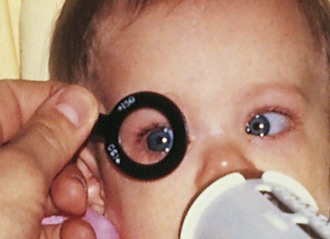
 to 5 years of age. Family histories of hypertropia, anisometropia, esotropia, and amblyopia are very common. The presence of uncorrected hyperopia causes the patient to accommodate or focus to obtain clear visual acuity. With accommodation, the synkinetic near response, which includes miosis, accommodation, and convergence of the eyes, occurs. If the fusion mechanism is unable to diverge the eyes to compensate for the excessive convergence accompanying the need to accommodate to correct for the patient’s hyperopia, esotropia results.
to 5 years of age. Family histories of hypertropia, anisometropia, esotropia, and amblyopia are very common. The presence of uncorrected hyperopia causes the patient to accommodate or focus to obtain clear visual acuity. With accommodation, the synkinetic near response, which includes miosis, accommodation, and convergence of the eyes, occurs. If the fusion mechanism is unable to diverge the eyes to compensate for the excessive convergence accompanying the need to accommodate to correct for the patient’s hyperopia, esotropia results.