Review of how too much or too little LH can affect an IVF cycle
14.2 Avoidance of Pre-cycle Oral Contraceptives
While most of our minimal stimulation in vitro fertilization (IVF) patients have diminished ovarian reserve and would not be expected to have hypogonadotropic hypogonadism, we take this lesson from nature to help us apply the importance of LH presence during our treatment protocols and to help us avoid iatrogenic low LH. One of the most common practices we see that can cause LH suppression is the use of pretreatment oral contraceptive pills.
For traditional IVF, oral contraceptive pills prior to stimulation may have many advantages. First, they help prevent ovarian cysts prior to stimulation start, reduce the risk for ovarian hyperstimulation syndrome, and may even result in better cycle outcomes in certain high-responder patients [7–9]. Second, they allow for follicular synchronization, which ultimately increases the chances that the follicles will grow at an even pace throughout stimulation. Third, the use of oral contraceptive pills allows fertility clinics to schedule IVF cycles according to facility and embryologist availability and thus adds to convenience for both the clinic and the patient. All of these reasons make the use of oral contraceptive pills leading up to IVF stimulation very reasonable for patients who are expected to be normal or high responders.
However, the use of long-term oral contraceptive pills for patients with diminished ovarian reserve needs to be avoided. Oral contraceptive use has been shown to decrease ovarian volume, antral follicle count, and anti-Mullerian hormone (AMH) levels [10]. Since patients with diminished ovarian reserve are already low on all of these factors, the implementation of oral contraceptives can have a large detrimental effect. In addition, oral contraceptives reduce endogenous DHEA-S, testosterone, and serum insulin-like growth factor (IGF-1) levels [11–13]. At a time when many patients are eager to take fertility supplements such as DHEA, testosterone, and growth hormone, we feel that a better strategy is to avoid suppression of those important cofactors. Oral contraceptives and, more specifically, the progestin component of oral contraceptives are known to suppress endogenous LH [14, 15]. The length of suppression after cessation of oral contraceptives can vary, but since IVF stimulation is usually started approximately 3–4 days after oral contraceptives have been stopped, it is logical to expect some ongoing effects during the early portion of the stimulation. Since the early part of the stimulation is critical for optimizing the number of follicles that can be recruited for the cycle, we recommend complete avoidance of oral contraceptives altogether just before starting the stimulation. Alternatively, we recommend the use of estrogen priming. As discussed in the minimal stimulation chapter, the short-term use of oral contraceptives for a few days may be needed in some clinical scenarios. However, any such use is always after estrogen priming and followed by estrogen priming again before starting stimulation.
14.3 Estrogen Priming as an Alternative to Pre-cycle Oral Contraceptives
Estrogen suppresses endogenous FSH, which allows for the same follicular synchronization that oral contraceptives provide. But, unlike progestin use, estrogen use does not cause profound LH suppression. Hence, its use prior to minimal stimulation IVF is beneficial. Our estrogen priming protocol consists of initiating estradiol 4 mg orally starting approximately 3–4 days after ovulation as confirmed by a rise in the serum progesterone level [16]. The oral estradiol is discontinued at the time of menses in preparation for IVF stimulation start on cycle day 2 or 3.
14.4 The Importance of LH Monitoring
When we initially began implementing minimal stimulation IVF for patients with diminished ovarian reserve, we did not monitor LH levels. Unfortunately, at that time (2012–2014), we had a premature ovulation rate of 12% (unpublished data). We have learned that women with diminished ovarian reserve and/or advanced reproductive age tend to have a higher risk for premature ovulation when compared to normal or high responders [17]. At the same time, the standard fixed or flexible GnRH antagonist protocols that are commonly used for the average patient are not applicable to our special patient population as mentioned in minimal stimulation IVF section.
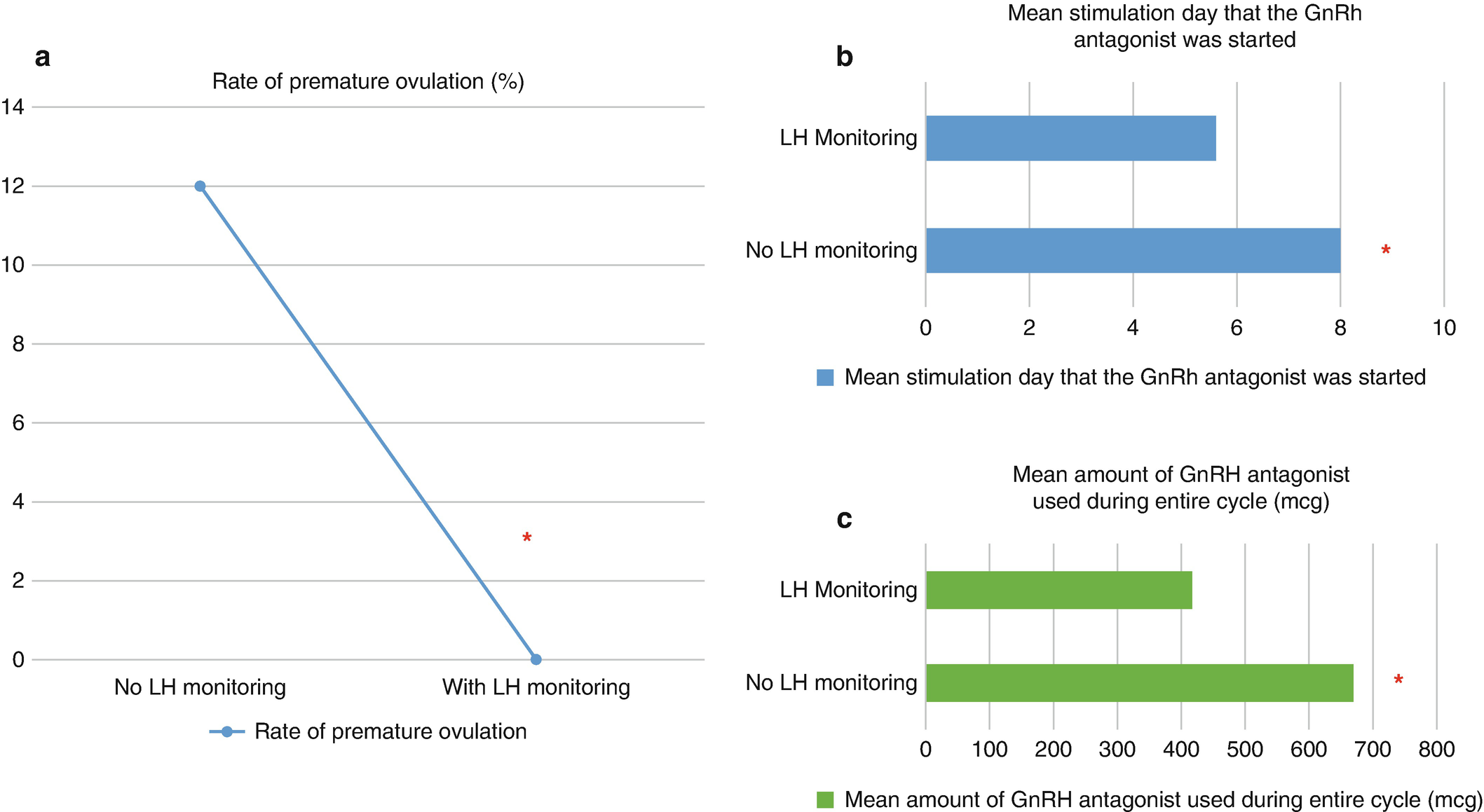
(a–c) Based on retrospective unpublished data collected at University of Texas Southwestern Medical Center, Dallas, Texas. Eighty-eight patients underwent 129 minimal stimulation IVF cycles, and they were divided between two groups: patients who did not have LH monitoring and patients who had LH monitoring during their minimal stimulation IVF cycle. (a) The premature ovulation rate was eliminated once LH monitoring was incorporated (p = 0.0017). (b) The addition of LH monitoring resulted in earlier initiation of the GnRH antagonist on average when compared to no LH monitoring (p < 0.0001). (c) The group with LH monitoring required roughly only 2/3 of the amount of GnRH antagonist when compared to the group that did not undergo LH monitoring (p < 0.0001)
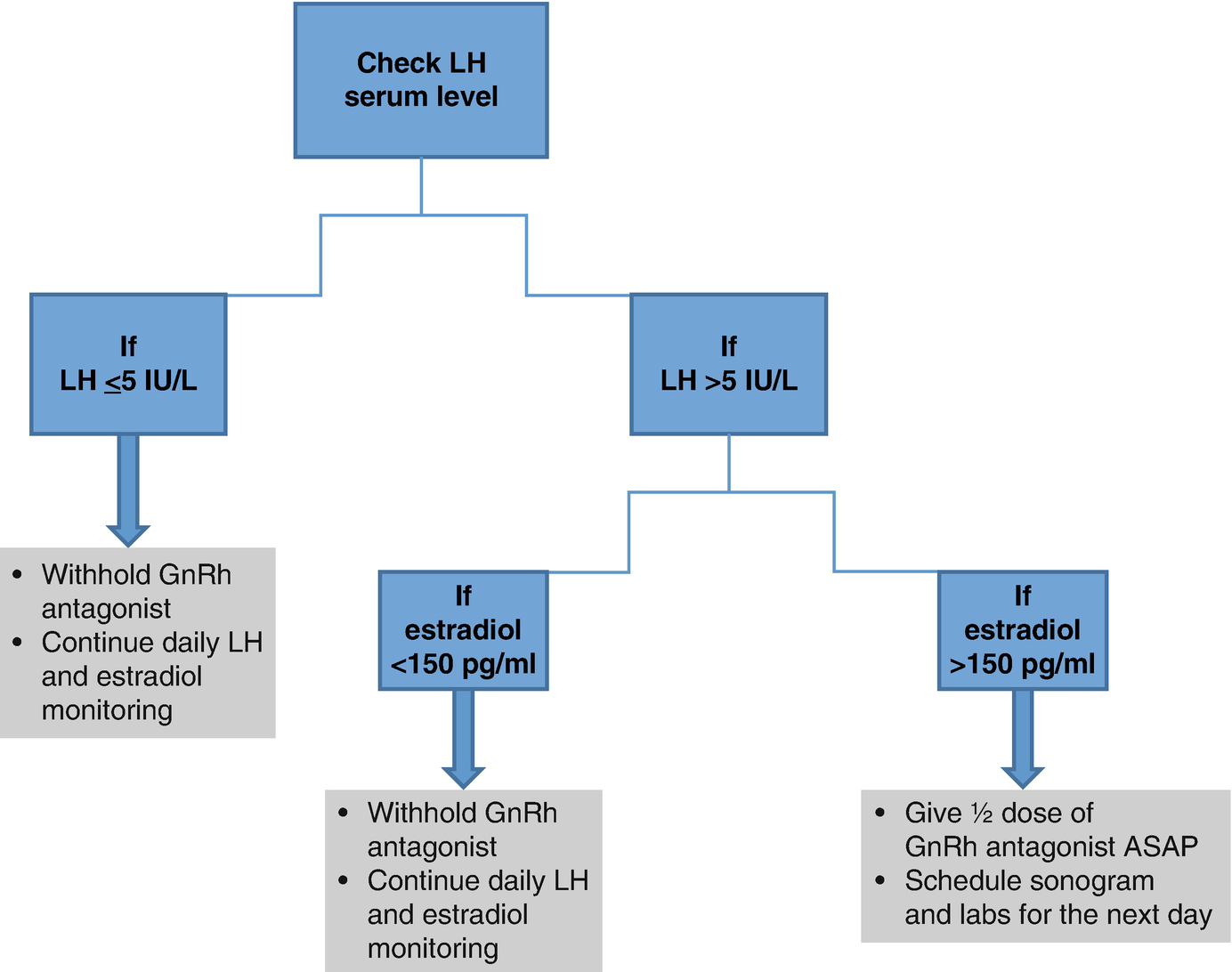
14.5 When to Initiate GnRH Antagonist Treatment

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


