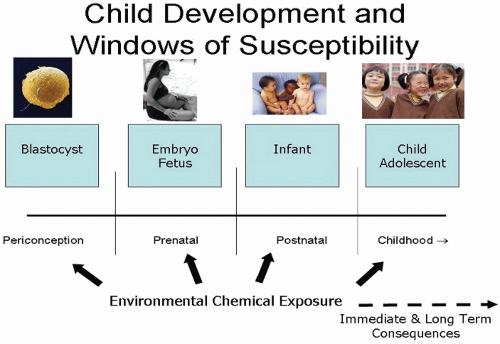
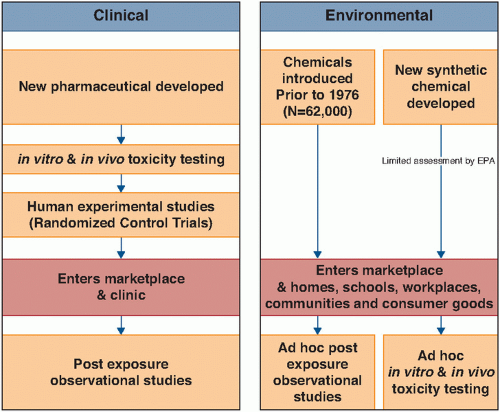
Types of Contaminants and Examples |
Sources and Exposure Circumstances |
Metals |
Mercury |
Occurs from energy production emissions and naturally. According to the U.S. EPA, coal-fired power plants are the largest current sources of mercury emissions in the country. Enters the aquatic food chain through a complex system. Primary exposure by consumption of contaminated seafood. |
Lead |
Occupational exposure occurs in battery manufacturing/recycling, smelting, car repair, welding, soldering, firearm cleaning/shooting, stained glass ornament/jewelry making; nonoccupational exposure occurs in older homes where lead-based paints were used, in or on some toys/children’s jewelry, water pipes, imported ceramics/pottery, herbal remedies, traditional cosmetics, hair dyes, contaminated soil, toys, costume jewelry. |
Organic compounds |
Solvents |
Used for cleaning, degreasing, embalming, refinishing and paint systems in a wide range of industries. Found in automotive products, degreasers, thinners, preservers, varnish and spot removers, pesticides (inert component), and nail polish. |
Ethylene oxide |
Occupational exposure to workers sterilizing medical supplies or engaged in manufacturing. |
Pentachlorophenol |
Wood preservative for utility poles, railroad ties, wharf pilings; formerly a multiuse pesticide. Found in soil, water, food, and breast milk. |
Bisphenol-A (BPA) |
Chemical intermediate for polycarbonate plastic and resins. Found in consumer products and packaging. Exposure through inhalation, ingestion, and dermal absorption. |
Polychlorinated biphenyls (PCBs) |
Used as industrial insulators and lubricants. Banned in the 1970s, but persistent in the aquatic and terrestrial food chains resulting in exposure by ingestion. |
Dioxins |
Dioxins and furans are multiple toxic chemicals formed by trash and waste incineration involving chlorine and, as categorized as a persistent organic pollutants (POPs), pervasive chemicals which bioconcentrate as they move up the food chain. Found in dairy products, meat, fish and shellfish. |
Perfluorochemicals (PFCs) |
PFCs are widely used man-made organofluorine compounds with many diverse industrial and consumer product applications. Examples are perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), which are used in the manufacture of nonstick Teflon and other trademark cookware products and in food-contact packaging to provide grease, oil, and water resistance to plates, food containers, bags, and wraps that come into contact with food. Persist in the environment. Occupational exposure to workers and general population exposure by inhalation, ingestion, and dermal contact. |
Polybrominated diphenyl ethers (PBDEs) |
Flame retardants that persist and bioaccumulate in the environment. Found in furniture, textiles, carpeting, electronic, and plastics. |
Di-(2 ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), di-n-butyl phthalate (DBP) |
Synthetically derived; phthalates are used in a variety of consumer goods such as medical devices, cleaning and building materials, personal care products, cosmetics, pharmaceuticals, food processing, and toys. Exposure occurs through ingestion, inhalation, and dermal absorption. |
Pesticides |
Applied in large quantities in agricultural, community, and household settings. In 2001, over 1.2 billion pounds of pesticide active ingredients were used in the United States. Pesticides can be ingested, inhaled, and absorbed by the skin. The pathways of pesticide exposure include food, water, air, dust, and soil. |
Chlorpyrifos |
Organophosphate pesticide used in agricultural production and for home pest control (home uses are now restricted). |
Dichlorodiphenyltrichloroethane (DDT)* |
Organochlorine insecticide, banned in the United States in the 1970s, is still used for malaria control overseas. Present in the food chain. |
Air contaminants |
Environmental tobacco smoke (ETS) Particulate matter (PM), ozone, lead Glycol ethers |
Burning of tobacco products. Exposure by inhalation from active or passive smoking. Sources include combustion of wood and fossil fuels, and industrial production. Exposure by inhalation. Used in enamels, paints, varnishes, stains electronics, and cosmetics. Occupational and general population exposure by inhalation, ingestion, and dermal contact. |
Note: Contaminants in italics are persistent and/or bioaccumulative. |
Adapted from Fox MA. Environmental contaminants and exposure. In: Woodruff TJ, Janssen JS, Guillette LJ Jr, et al, eds. Environmental Impacts on Reproductive Health and Fertility. New York: Cambridge University Press; 2010. |
|