Chapter 534 Obstruction of the Urinary Tract
Urinary tract obstruction can result from congenital (anatomic) lesions or can be caused by trauma, neoplasia, calculi, inflammatory processes, or surgical procedures, although most childhood obstructive lesions are congenital. Obstructive lesions occur at any level from the urethral meatus to the calyceal infundibula (Table 534-1). The pathophysiologic effects of obstruction depend on its level, the extent of involvement, the child’s age at onset, and whether it is acute or chronic.
Table 534-1 TYPES AND CAUSES OF URINARY TRACT OBSTRUCTION
| LOCATION | CAUSE |
|---|---|
| Infundibula | |
| Renal pelvis | |
| Ureteropelvic junction | |
| Ureter | |
| Bladder outlet and urethra |
Etiology
Ureteral obstruction occurring early in fetal life results in renal dysplasia, ranging from multicystic kidney, which is associated with ureteral or pelvic atresia (see Fig. 531-2), to various degrees of histologic renal cortical dysplasia that are seen with less severe obstruction. Chronic ureteral obstruction in late fetal life or after birth results in dilation of the ureter, renal pelvis, and calyces, with alterations of renal parenchyma ranging from minimal tubular changes to dilation of Bowman’s space, glomerular fibrosis, and interstitial fibrosis. After birth, infections often complicate obstruction and can increase renal damage.
Clinical Manifestations
Obstruction of the urinary tract generally causes hydronephrosis, which typically is asymptomatic in its early phases. An obstructed kidney secondary to a ureteropelvic junction (UPJ) or ureterovesical junction obstruction can manifest as a mass or cause upper abdominal or flank pain on the affected side. Pyelonephritis can occur because of urinary stasis. An upper urinary tract stone can occur, causing abdominal and flank pain and hematuria. With bladder outlet obstruction, the urinary stream may be weak; urinary tract infection (UTI; Chapter 532) is common. Many of these lesions are identified by antenatal ultrasonography; an abnormality involving the genitourinary tract is suspected in as many as 1/100 fetuses.
Diagnosis
Imaging Studies
Renal Ultrasonography
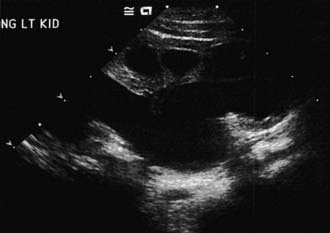
The presence of a dilated urinary tract is the most common characteristic of obstruction. Hydronephrosis is a common ultrasonographic finding (Fig. 534-1). Dilation is not diagnostic of obstruction and can persist after surgical correction of an obstructive lesion. Dilation can result from vesicoureteral reflux, or it may be a manifestation of abnormal development of the urinary tract, even when there is no obstruction. Renal length, degree of caliectasis and parenchymal thickness, and presence or absence of ureteral dilation should be assessed. Ideally, the severity of hydronephrosis should be graded from 1 to 4 using the Society for Fetal Urology grading scale (Table 534-2). The clinician should ascertain that the contralateral kidney is normal, and the bladder should be imaged to see whether the bladder wall is thickened, the lower ureter is dilated, and bladder emptying is complete. In acute or intermittent obstruction, the dilation of the collecting system may be minimal and ultrasonography may be misleading.
Table 534-2 SOCIETY FOR FETAL UROLOGY GRADING SYSTEM FOR HYDRONEPHROSIS
| RENAL IMAGE | ||
|---|---|---|
| GRADE OF HYDRONEPHROSIS | Central Renal Complex | Renal Parenchymal Thickness |
| 0 | Intact | Normal |
| 1 | Slight splitting | Normal |
| 2 | Evident splitting, complex confined within renal border | Normal |
| 3 | Wide splitting pelvis dilated outside renal border, calyces uniformly dilated | Normal |
| 4 | Further dilatation of pelvis and calyces (calyces may appear convex) | Thin |
After Maizels M, Mitchell B, Kass E, et al: Outcome of nonspecific hydronephrosis in the infant: a report from the registry of the Society for Fetal Urology, J Urol 152:2324–2327, 1994.
Radioisotope Studies
In a MAG-3 diuretic renogram, a small dose of technetium-labeled MAG-3 is injected intravenously (Figs. 534-2 and 534-3). During the first 2-3 min, renal parenchymal uptake is analyzed and compared, allowing computation of differential renal function. Subsequently, excretion is evaluated. After 20-30 min, furosemide 1 mg/kg is injected intravenously, and the rapidity and pattern of drainage from the kidneys to the bladder are analyzed. If no obstruction is present, half of the radionuclide should be cleared from the renal pelvis within 10-15 min, termed the half-time (t1/2). If there is significant upper tract obstruction, the t1/2 usually is >20 min. A t1/2 of 15-20 min is indeterminate. The images generated usually provide an accurate assessment of the site of obstruction. Numerous variables affect the outcome of the diuretic renogram. Newborn kidneys are functionally immature, and, in the first month of life, normal kidneys might not demonstrate normal drainage after diuretic administration. Dehydration prolongs parenchymal transit and can blunt the diuretic response. Giving an insufficient dose of furosemide can result in inadequate drainage. If vesicoureteral reflux is present, continuous bladder drainage is mandatory to prevent the radionuclide from refluxing from the bladder into the dilated upper tract, which would prolong the washout phase.
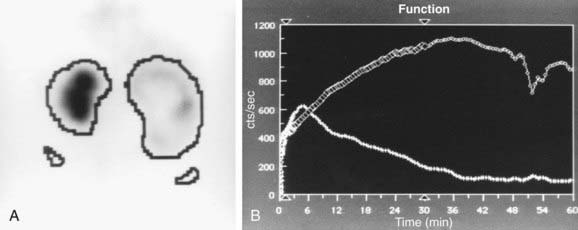
Figure 534-2 Same patient as in Figure 534-1. 6 wk old, MAG-3 diuretic renogram. The right kidney is on the right side of the image. A, Differential renal function: left kidney 70%, right kidney 30%. B, After administration of furosemide, drainage from the left kidney was normal and drainage from the right kidney was slow, consistent with right ureteropelvic junction obstruction. Pyeloplasty was performed on the right kidney.
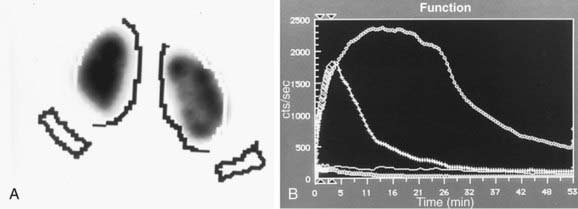
Figure 534-3 Same patient as in Figure 534-1. A, MAG-3 diuretic renogram at 14 mo of age shows equal function in the two kidneys. B, Prompt drainage after the administration of furosemide.
Magnetic Resonance Urography
MR urography is also used to evaluate suspected upper urinary tract pathology. The child is hydrated and given intravenous furosemide. Gadolinium-DTPA is injected and routine T1-weighted and fat-suppressed fast spin-echo T2-weighted imaging is performed through the kidneys, ureters, and bladder. This study provides superb images of the pathology, and methodology permits assessment of differential renal function and drainage (Fig. 534-4). There is no radiation exposure; younger children need sedation or anesthesia. It is used primarily when renal sonography and nuclear imaging fail to delineate complex pathology.
Ancillary Studies
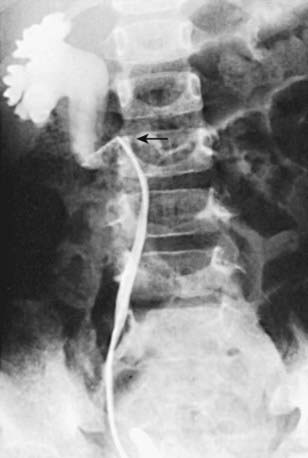
In unusual cases, an antegrade pyelogram (insertion of a percutaneous nephrostomy tube and injection of contrast agent), can be performed to assess the anatomy of the upper urinary tract. This procedure usually requires general anesthesia. In addition, an antegrade pressure-perfusion flow study (Whitaker test) may be performed, in which fluid is infused at a measured rate, usually 10 mL/min. The pressures in the renal pelvis and the bladder are monitored during this infusion, and pressure differences exceeding 20 cm H2O suggest obstruction. In other cases, cystoscopy with retrograde pyelography provides excellent images of the upper urinary tract (Fig. 534-5).