Obstetric Anesthesia and Analgesia: Effects on the Fetus and Newborn
Judith Littleford
Many drugs and various techniques have been used to provide anesthesia and analgesia for surgery during pregnancy, for labor and delivery, and for breastfeeding. The following quote, which refers to the first administration of inhalational analgesia in childbirth, is as relevant to the practice of obstetric anesthesia today as it was in 1847, “It will be necessary to ascertain anesthesia’s precise effect, both upon the action of the uterus and on the assistant abdominal muscles; its influence, if any, upon the child; whether it has the tendency to hemorrhage or other complications” (1). Between the mid-1800s and 1950s, descriptive reports of the presumed effect of maternally administered medication on the fetus and newborn appeared sporadically in the literature. Two developments eventually encouraged physicians to acknowledge the potential problems associated with placental transmission of anesthetic drugs:
Recognition that morphine, a popular ingredient of patent medicines, was addictive, and that signs of withdrawal could be identified in the fetus (violent fetal movements and/or sudden fetal death) when the mother’s heavy opioid use was decreased.
Confirmation of the structure and dynamic function of the placenta and demonstration of the presence of chloroform in the umbilical blood of neonates.
In 1952 the pioneering work of anesthesiologist Virginia Apgar converted an intangible phenomenon, the clinical condition of a newly born baby, into a formally defined measurement (2). Thereafter, the well-being of the infant became a major criterion for evaluation of the obstetric and anesthetic management of pregnant women.
This chapter introduces the neonatal practitioner to the clinical aspects of obstetric anesthesia and analgesia and examines their effects on the fetus and newborn.
EVALUATION OF WELL-BEING
Several methods of evaluation have been adopted into common usage as anesthesiologists attempt to separate out the fetal/neonatal effects of their interventions from concomitant medical and nursing management, and from the influence of preexisting maternal conditions.
The Apgar Score
The Apgar score is a convenient method of reporting the status of the newborn and the effectiveness of resuscitation. It rates each of five physical signs traditionally used by anesthesiologists to monitor a patient’s condition: heart rate, respiratory effort, muscle tone, reflex irritability, and color. Apgar demonstrated that her score was sufficiently sensitive to detect differences among newborns whose mothers had received spinal versus general anesthesia (GA) for cesarean section (2).
Elements of the score are partly dependent on the physiologic maturity of the infant. Likewise, neonatal conditions such as bradyarrhythmias affect heart rate, while infection, neuromuscular conditions, and certain medications affect respiratory effort and tone. Additional information is required to interpret Apgar scores meaningfully in infants receiving ongoing resuscitation.
Wider application has resulted in incorrect use of the Apgar score to evaluate infant morbidity/mortality and to predict the occurrence of intrapartum events and long-term disability. Apgar scores at 1 and 5 minutes correlate poorly with both cause and neurologic outcome. A joint statement from the American Academy of Pediatrics (AAP) and American College of Obstetricians and Gynecologists emphasizes the appropriate use of the Apgar Score (3).
When used as originally intended, the Apgar score remains a valuable tool to assess the condition of the infant at birth (4), but it is not specific for the effects of anesthesia on the newborn.
When used as originally intended, the Apgar score remains a valuable tool to assess the condition of the infant at birth (4), but it is not specific for the effects of anesthesia on the newborn.
Umbilical Cord Blood Gas Analysis
Cord blood gas analysis is the gold standard for assessing fetal acid-base status and uteroplacental function at birth. Umbilical artery pH, base excess, and pCO2 reflect fetal and immediate neonatal condition whereas umbilical vein values reflect maternal acid-base status and placental function.
“Normal” values vary depending on the definition of normality and the influence of factors (e.g., altitude, parity, breech vaginal delivery, and duration of labor) on the population studied (5). Helwig and associates (6) retrospectively examined the records of 15,000 vigorous newborns with a 5-minute Apgar score of >7. Median umbilical artery values, with the 2.5th percentile value in parentheses, were pH 7.26 (7.10) and base excess -4 mmol/L (-11 mmol/L). The means ± 2 standard deviations were similar. The generally accepted lower limit of normal umbilical artery pH extends to 7.10 and base excess to -12 mmol/L (5,7).
Values for pH, pCO2, and base excess also vary with differences in sampling technique. Preanalytical error can be introduced if the cord is not clamped immediately, there is an excess quantity of heparin in relation to the amount of blood collected, air is present in the syringe, or the sample is kept at room temperature for longer than 15 minutes (8).
The supply of oxygen and the removal of volatile (CO2) and fixed (e.g., lactate) acids by the placenta for excretion by the maternal lungs and kidneys, respectively, allow the fetus to maintain acid-base balance within a narrow range. Interruption of these processes can lead to acidemia in the fetus. In general, respiratory acidosis alone is not associated with newborn complications; rather, it reflects a sudden decrease in uteroplacental or umbilical perfusion such as placental abruption or cord prolapse immediately preceding delivery. Base excess values have greater usefulness than pH values because base excess does not change significantly with respiratory acidosis and demonstrates linear, rather than logarithmic, correlation to the degree of metabolic acidosis. Umbilic artery base excess is the most direct measure of fetal metabolic acidosis. Human and animal studies have confirmed normal values before and during labor and rates of base excess change in relation to events causing fetal hypoxemia (maternal hypoxemia, umbilical cord occlusion, and reduced uterine blood flow) (7).
The process of normal labor and delivery without anesthetic intervention stresses the fetus such that mild acidosis develops in almost all labors (9). Reynolds and associates (10) recently completed a metanalysis comparing epidural with systemic opioid analgesia to determine the effect of these anesthetic interventions on acid-base status at birth. They concluded that epidural analgesia was associated with an improvement in base excess, suggesting that placental exchange is well preserved in association with this technique.
While umbilical artery pH, base excess, and pCO2 are considered sensitive and objective indicators of fetal hypoxia during labor, the results represent a “snapshot” of fetal status and depict the mixed effluent of all fetal tissues. Cord gases do not distinguish between primary fetal pathologic conditions, fetal effects of maternal conditions (e.g., acid-base disorders), or the influence of inadequate placental blood flow. They also do not indicate in which direction the condition of the fetus is moving, or at what rate, nor do they reflect events that occurred remote from delivery.
Fetal Scalp Blood Gas Analysis
This method is advocated to improve the specificity of fetal heart rate monitoring (11) but has not been used by anesthesiologists to measure the fetal effects of their interventions.
Evaluation of Newborn Neurobehavior
Brazelton, Scanlon, and Amiel-Tison assessed the effect of anesthetic medications on the neurobehavior of term, healthy newborns (12,13,14). Their belief was that central nervous system depression from drugs administered to the mother during labor and delivery could be distinguished from effects associated with perinatal asphyxia and trauma at birth. Several review articles have described these tests in detail and compared the Brazelton Neonatal Behavioral Assessment Scale (NBAS) (12) and Early Neonatal Neurobehavioral Scale (ENNS) (13) to the Neurologic and Adaptive Capacity Score (NACS) (14).
Researchers in obstetric anesthesia have tended to favor the NACS. It emphasizes muscle tone, avoids aversive stimuli, can be completed quickly, and is considered easier to learn than the NBAS and ENNS. The 20-item instrument is organized into two scales, adaptive capacity and neurologic assessment. The latter is further divided into four subscales, passive tone, active tone, primary reflexes, and general neurologic status assessment. A systematic review of the literature regarding use of NACS in obstetric anesthesia research concluded that reliability and validity evaluations of the tool were lacking, and it was unclear whether NACS could detect the existence of subtle neurobehavioral effects (15). The topic was the subject of an editorial that examined the widespread acceptance of this test notwithstanding the criticism that surrounded its initial publication (16). Subsequently, it has been determined that NACS has poor reliability when used to detect the effects of intrapartum drugs and other interventions on the neonate (17).
The NBAS is a much more detailed examination that may be more sensitive to environmental influences on behavior and reflect more accurately the capabilities of the neonate (18). Early studies involving concentrated epidural solutions tended to show that babies delivered to medicated mothers were less alert, had poorer muscle tone, and had difficulty habituating to repeated stimuli. This finding
is much less likely to occur with modern obstetric analgesia (19), where emphasis is placed on administering the lowest effective dose of medication or combination of medications needed to achieve the desired maternal effect and reduce the likelihood of adverse influences on the fetus/newborn.
is much less likely to occur with modern obstetric analgesia (19), where emphasis is placed on administering the lowest effective dose of medication or combination of medications needed to achieve the desired maternal effect and reduce the likelihood of adverse influences on the fetus/newborn.
Electronic Fetal Monitoring
Electronic fetal monitoring, one technique in the overall strategy of intrapartum fetal surveillance (20,21), aims to improve outcomes by identifying fetuses with hypoxic acidemia at a point when the process is still completely reversible by intrauterine resuscitation or expedited delivery. The fetal heart rate (FHR), including variability, accelerations, and decelerations, if any occur, is recorded electronically on a paper trace. A reactive (normal or reassuring) cardiotocogram (CTG) is defined by the presence of accelerations. Baseline FHR normally ranges between 110 and 160 beats per minute. Reduced variability and the presence of decelerations are abnormal findings.
A systematic review of studies comparing the efficacy and safety of routine continuous electronic fetal monitoring versus intermittent auscultation (IA) of the FHR reveals the former to be associated with a significantly higher rate of cesarean and operative vaginal delivery (22). In the antecedent Cochrane review of this same topic, the incidence of GA to facilitate obstetric intervention was also increased. This aspect of management was not examined in the current review. The only significant benefit of the use of routine, continuous fetal monitoring was in the reduction of neonatal seizures; otherwise, neither IA nor continuous monitoring has been shown to decrease morbidity or mortality (22).
Difficulty in the visual interpretation of CTG patterns during labor can result in unnecessary operative intervention, while some significant changes go unrecognized. Computerized CTG systems, which do not rely on observer reading of the FHR tracing, are more accurate and reliable. Evaluation of the FHR pattern is given online continuously, and warnings are displayed if there is signal loss, reduction in fetal movements, or an abnormally flat or decelerative trace (23). Retrospective analysis of several thousand records enabled investigators to conclude that the most reliable single parameter of fetal condition was variability (short- and long-term). Absence of accelerations, presence of decelerations, decrease in the number of movements, and changes in baseline FHR all occurred occasionally in normal fetuses (24).
Recently, automatic ST segment and T-wave analysis of the fetal electrocardiogram (recorded continuously from the fetal scalp electrode used for FHR measurement) has been combined with conventional CTG. Elevation or depression of the ST segment occurs when the fetus is exposed to a hypoxic stress. When CTG is accompanied by computerized analysis of the ST segment, the number of operative deliveries for the indication of “fetal distress” can be reduced without jeopardizing fetal outcome (24). This technique of fetal surveillance improves the practitioner’s ability to identify cases of significant fetal hypoxia necessitating obstetric intervention, thus reducing the risk of an umbilical cord arterial metabolic acidosis at birth (25).
Anesthesiologists have used continuous fetal CTG recordings to evaluate the effect of maternal analgesia on FHR and variability. Solt and associates (26) demonstrated that intrapartum intravenous (IV) administration of meperidine (pethidine) 50 mg and promethazine 25 mg resulted in decreased variability and fewer accelerations on computerized CTG for the duration of their 40-minute recording. It is unclear whether the effect can be attributed to one of the two drugs or the combination, although this is a typical fetal response to systemic maternal opioid administration.
A randomized study was conducted to examine the effect of continuous epidural anesthesia with or without narcotic on intrapartum FHR characteristics as measured by computer analysis. The narcotic epidural consisted of an initial bolus of 10-12 mL 0.125% bupivacaine with fentanyl 50 μg, followed by continuous bupivacaine 0.125% with fentanyl 1.7μg/mL infusion. The nonnarcotic epidural was initiated with a bolus of 10-12 mL bupivacaine 0.25% and followed by a bupivacaine 0.125% infusion. Investigators found no difference in pre- and postepidural baseline FHR, accelerations, or variability between the groups (27). These solutions are considerably less concentrated than those used 10-20 years ago and are in keeping with modern obstetric anesthesia practice, which aims to reduce motor block by using dilute local anesthetic (LA) plus narcotic epidural solution combinations.
In a double-blind randomized study of bolus epidural opioid effect on FHR variability, butorphanol 2 mg, fentanyl 50 μg, sufentanil 15 μg, or saline in combination with bupivacaine 0.25% did not change FHR short- or long-term variability (28).
FHR decreases in response to compression of the fetal head during passage through the birth canal and in response to umbilical cord compression or reduced uterine blood flow secondary to maternal hypotension or prolonged uterine contraction. This bradycardia is vagally mediated.
Biophysical Profile Score
This ultrasound-based method combines measures of acute biophysical variables, fetal breathing, heart rate accelerations, gross body movements, and fetal tone with amniotic fluid volume (29). The first four variables reflect acute fetal condition, whereas the last variable reflects chronic fetal condition. The observation period lasts 30 minutes because fetuses are known to sleep for intervals lasting approximately 30 minutes. When normal (≥8 out of a possible 10), the biophysical profile score (BPS) is a direct, reliable, and accurate measure of normal tissue oxygenation. A normal score is never associated with an abnormal fetal pH. Scores ≤6/10 during routine antepartum evaluation indicate the amount of oxygen
delivered to target organs is insufficient to maintain function. The lower the score, the greater the likelihood that central acidemia is present (30).
delivered to target organs is insufficient to maintain function. The lower the score, the greater the likelihood that central acidemia is present (30).
Placental transfer to the fetus of maternally administered intramuscular (i.m.) narcotic medication (diamorphine 10 mg or morphine 10 or 15 mg administered with dimenhydrinate) resulted in transiently reduced fetal activity and, consequently, a lower BPS for the duration of the drug effect (31,32). A small dose of IV fentanyl (50 μg) given in early labor was associated with abolishment of fetal breathing at 10 minutes postdosing, fewer body movements, and reduced variability (33). The effect lasted approximately 30 minutes, in keeping with the pharmacodynamic profile of fentanyl. This should be taken into account when fentanyl is administered close to the time of delivery. In this study, none of the neonates required resuscitation, and all had umbilical artery pH values >7.2.
Fetal Pulse Oximetry
Fetal oxygen saturation monitoring by pulse oximetry (SpO2) is poised to become the most significant advance for intrapartum fetal assessment in recent years. It relies on established, noninvasive technology and is based on the following assumptions: fetal well-being is dependent on perfusion of vital organs with oxygenated blood; SpO2 correlates with saturation as measured by blood gas analysis; and a critical threshold for SpO2 exists, above which the fetus will be nonacidemic and below which, acid-base decompensation may occur (34).
Based on animal and human data, fetal SpO2 values ≥30% can be considered reassuring, whereas SpO2 values <30% may be associated with acidosis. Low SpO2 values warrant further assessment if they persist for periods of >2 minutes and necessitate intervention (intrauterine resuscitation or expedited delivery) if they persist for >10 minutes (34,35). The predictive value of intrapartum fetal SpO2 compares favorably with fetal scalp blood analysis (36). The former has the advantage of being a continuous monitoring technique.
The United States Food and Drug Administration approved the Nellcor N-400/FS14 fetal pulse oximeter for clinical use in 2000 as an adjunct to electronic fetal monitoring in the presence of a nonreassuring tracing (Fig. 16-1). At present, this technology is limited to singleton, term fetuses in the vertex position.
Results of studies designed to investigate the effects on fetal oxygen saturation of administering epidurals to healthy parturients are beginning to appear in the literature. Neither an initial epidural bolus of 15 mL of ropivacaine 0.1% with sufentanil 10 μg or intermittent repeat plain ropivacaine boluses affected SpO2 in healthy fetuses (37). In a study designed to account for the possible influence of maternal position, diastolic blood pressure, and preexisting FHR pattern, SpO2 values were not affected by boluses of dilute epidural infusion solutions but did decrease with bolus administration of more concentrated LA at epidural insertion or top-up (38). Other than to comment on the variety of combinations and strengths of analgesic agents, details of the epidural solutions were not contained in the report. Further clinical trials are needed to assess and characterize the effect of all types of anesthetic intervention on fetal SpO2 during normal and abnormal labor, in addition to various maternal conditions associated with fetal compromise (e.g., pregnancy-induced hypertension).
Fetal Doppler
Flow velocity waveforms from maternal vessels (uterine arteries), placental circulation (umbilical arteries), and fetal systemic vessels (e.g., middle cerebral artery), collectively known as Doppler evaluation, provide prognostic and diagnostic detail about placentation and fetal adaptation (39).
Regulation of the circulation is a complex fetal behavior, influenced by gestational age and the maternal environment. Under normal circumstances, the reduction in sympathetic tone created by epidural analgesia does not affect Doppler flow characteristics of either the uterine or umbilical artery vessels because the spiral arterioles are maximally dilated and the fetoplacental circulation is stable and tolerant of environmental changes. However, epidural analgesia has been shown to improve uteroplacental perfusion and effectively reduce maternal blood pressure in laboring patients with pregnancy-induced hypertension (40). This offers potential benefits for both the fetus and mother: when uteroplacental perfusion improves, fetal oxygenation and acid-base balance improve, and when blood pressure is restored to normal levels, the risk of vascular accidents and organ damage is reduced.
Static Charge-Sensitive Bed
A new method for long-term monitoring of respiration, heart rate, cardiac function, and body movements in newborn infants was presented in 1984, and possible clinical
applications were discussed (41). The technology has been used to evaluate neonatal sleep states following unmedicated vaginal delivery or elective cesarean section delivery under spinal anesthesia (42) and, in combination with SpO2 and ECG recording, to examine the influence on the newborn baby of maternal fentanyl analgesia in labor (43).
applications were discussed (41). The technology has been used to evaluate neonatal sleep states following unmedicated vaginal delivery or elective cesarean section delivery under spinal anesthesia (42) and, in combination with SpO2 and ECG recording, to examine the influence on the newborn baby of maternal fentanyl analgesia in labor (43).
In the first study, mode of delivery did not affect sleep state distribution during the first day of life (42). Vaginally born neonates had fewer body movements and more episodes of SpO2 <95% in the first 24 hours after birth. This result surprised the investigators since babies born by cesarean section are known to develop respiratory problems more often than infants who are delivered vaginally. In the second study, fentanyl (50 μg of IV bolus q5min until pain relief, then fentanyl patient-controlled analgesia) was compared with paracervical block (10 mL of bupivacaine 0.25%) in a prospective, randomized fashion. The trial was interrupted after enrolment of the twelfth healthy, term newborn because there was a significant decrease in SpO2 to 59% in one of the babies. Interestingly, the SpO2 improved with naloxone administration even though later analysis showed the concentration of fentanyl in the umbilical vein to be below the detection limit of the assay. Intrapartum electronic fetal monitoring did not reveal any difference in variability or heart rate between groups. As well, Apgar scores and analyses of umbilical artery pH were similar. The SpO2 values were lower and the percentage of minimum SpO2 values between 81% and 90% were more prevalent in the fentanyl group. The static charge-sensitive bed (SCSB) method proved sensitive enough to detect lower heart rates and less quiet sleep in the fentanyl group, suggesting a salutary effect of the opioid on delivery stress (43).
Summary
The nature of the association of anesthetic medications and interventions is complex and can be confounded by a myriad of factors. As yet, there is no one test that clearly separates effects on the fetus/newborn, if any, of maternally administered medication during labor and delivery, although newer technologies show some promise.
PAIN MANAGEMENT
For most women, childbirth is likely one of the most painful events in their lifetimes. There are both physiologic and psychologic aspects to pain and its management (44).
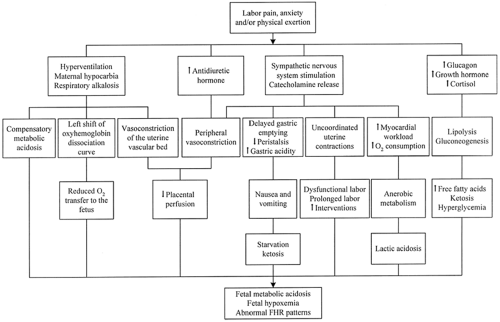
Labor pain evokes a generalized neuroendocrine stress response that has widespread physiologic effects on the parturient and fetus (45). The neuroendocrine model, presented in Figure 16-2, examines the potential detrimental consequences of untreated pain. The sequelae of hyperventilation, secretion of stress-related hormones, and increased oxygen consumption can be prevented, obtunded, or abolished by central neuraxial blockade (epidural or spinal anesthesia).
Research in humans supports elements of this model (46), but studies are not necessarily designed to consider the effects of simultaneously occurring care practices on these same physiologic responses. “This critique is needed because it is somewhat counterintuitive that the procreative physiologic process of labor and birth would by nature have detrimental effects on a healthy mother and fetus” (47). An example of a concurrent care practice is the administration of isotonic “sport drinks” versus water only during labor (48). Sports drinks were shown to prevent the development of maternal ketosis without increasing gastric volume, although there was no difference between the groups in neonatal outcome.
Labor Pain: Implications for the Fetus
Neural pathways and neurochemical systems involved in pain perception are functional from mid-gestation and are well developed by the third trimester (49,50). Gitau and associates (51) conducted a parallel study of the fetal and maternal hormonal responses to fetal blood transfusion. They confirmed that the fetus mounts a hypothalamic-pituitary-adrenal response to transfusion via the intrahepatic vein, which involves piercing the fetal trunk, but not to transfusion in the umbilical vein at the placental cord insertion, which has no sensory innervation. The rise in fetal cortisol and endorphin occurred independently of the maternal reaction. Pretreatment of the fetus with fentanyl for this same procedure attenuated the rise in β-endorphin (52).
Hormonal stress responses do not provide a direct index of pain. While it is true that a rise in cortisol and endorphin is seen as a consequence of painful stimuli in children, other nonpainful situations (e.g., exercise) are also associated with an increase in the levels of these hormones. Nonetheless, editorial review of Fisk’s fentanyl pretreatment study suggests that fetal analgesia should be given during invasive in utero procedures (53).
At present, there is no literature on fetal “pain” during labor or delivery.
Labor and Delivery Pain: Implications for the Mother
Visceral pain predominates during the first stage of labor. Nociceptive information arising from uterine contractions, distention of the lower uterine segment, and cervical dilation is relayed in C afferent fibers to the dorsal horn of the spinal cord at the T10 to L1 levels. As labor progresses, a mixture of visceral and somatic (A-delta fibers) pain results from traction on the pelvic floor structures surrounding the vaginal vault, and eventually from distention and stretch of the vagina and perineum (L2-S1). Delivery pain (Stage II) is somatic in nature and transmitted along the pudendal nerve (S2-4). Synaptic input at the dorsal horn, mediated by neurotransmitters and chemicals (e.g., excitatory amino acids), is relayed via the spinothalamic tract to higher centers including the reticular formation, hypothalamus, and limbic system. Dorsal horn neurons also initiate segmental spinal reflexes. Descending spinal tracts, endogenous opioids, and other inhibitory systems modulate
nociception centrally in the spinal cord. The neural mechanism of labor shares features with other forms of acute pain (54).
nociception centrally in the spinal cord. The neural mechanism of labor shares features with other forms of acute pain (54).
To view labor pain only as a neuroendocrine, sensory experience is limiting and undermines the complexity of this phenomenon (47). Pain is just one component of the totality of the labor and birth experience. Assisting women to cope with the affective or distress components of labor and birth in a supportive environment has been shown to reduce the need for pain-relieving drugs, decrease the incidence of operative delivery, result in higher Apgar scores, and improve breastfeeding success (44,55).
Practice guidelines, including a section devoted to specific analgesia techniques, have been developed to enhance the quality of anesthetic care for obstetric patients (56). For the obstetrician, analgesia options are outlined in a practice bulletin that was written to facilitate communication with patients and anesthesia and neonatology colleagues (57).
The management of pain and anxiety in labor is a worthwhile goal whether the techniques used are nonpharmacologic, pharmacologic, or include a combination of both. The choice depends on patient preferences, medical status of the mother and fetus, progress of labor, and resources available at the facility for pain management and treatment of potential complications.
ANALGESIC TECHNIQUES FOR LABOR: EFFECTS ON THE FETUS AND NEWBORN
Analgesia refers to pain relief without loss of consciousness. Regional analgesia denotes partial sensory blockade in a specific area of the body, with or without partial motor blockade. The term neuraxial analgesia pertains to the administration of pain-relieving medications using caudal, spinal, and/or epidural techniques.
Not all methods of pain relief are available or desirable in all centers, and certain methods are more popular in different parts of the world (58).
Nonpharmacologic Methods
Proponents of nonpharmacologic methods claim that these methods reduce requirements for analgesia during the first stage of labor. This does not necessarily imply that women who use these techniques have less pain, rather that they are able to cope with labor using less analgesia.
In a systematic review of comfort measures, Simkin and O’Hara (59) commented on five methods scientifically evaluated for their effectiveness in reducing indicators of labor pain. This review also mentioned pain-relatedoutcomes
such as obstetric interventions and duration of labor. Continuous labor support was associated with a decrease in duration of labor, requests for analgesia, rates of instrumental and cesarean deliveries, and occurrence of lower Apgar scores. The use of baths offered temporary pain relief and was considered safe provided water temperatures were maintained at or below maternal body temperature and that immersion duration was controlled. Perinatal morbidity and mortality did not increase, even if membranes were ruptured. The authors concluded that there had been insufficient study to provide clear conclusions regarding touch/massage, although emotional and physical relief was demonstrated with this intervention. Intradermal water blocks were effective in reducing severe back pain, and one randomized study reported a decrease in cesarean deliveries. Lastly, maternal movement and positioning was reported to impact pain relief in labor and impact several variables related to fetal and neonatal well-being. In this systematic review, no trials compared a policy of freedom to move spontaneously with a policy of restriction to a bed for outcomes such as comfort, labor progress, or fetal welfare. Mechanisms by which dystocia may be prevented or corrected through the use of maternal positioning have been discussed elsewhere (60).
such as obstetric interventions and duration of labor. Continuous labor support was associated with a decrease in duration of labor, requests for analgesia, rates of instrumental and cesarean deliveries, and occurrence of lower Apgar scores. The use of baths offered temporary pain relief and was considered safe provided water temperatures were maintained at or below maternal body temperature and that immersion duration was controlled. Perinatal morbidity and mortality did not increase, even if membranes were ruptured. The authors concluded that there had been insufficient study to provide clear conclusions regarding touch/massage, although emotional and physical relief was demonstrated with this intervention. Intradermal water blocks were effective in reducing severe back pain, and one randomized study reported a decrease in cesarean deliveries. Lastly, maternal movement and positioning was reported to impact pain relief in labor and impact several variables related to fetal and neonatal well-being. In this systematic review, no trials compared a policy of freedom to move spontaneously with a policy of restriction to a bed for outcomes such as comfort, labor progress, or fetal welfare. Mechanisms by which dystocia may be prevented or corrected through the use of maternal positioning have been discussed elsewhere (60).
Systemic Opioids
From the maternal perspective, efficacy and incidence of side effects with systemic opioid analgesia is largely dose- rather than drug-dependent. There is little evidence to suggest one agent is intrinsically superior. Most often, the choice is based on institutional tradition or personal preference.
Opioids may affect the fetus directly as a result of placental transfer and/or indirectly, for example, by altering maternal minute ventilation or uterine tone. As a group, these low-molecular-weight drugs are lipid-soluble weak bases (61) that readily cross the placenta. This implies that maternal to fetal concentration gradients are important; only free, not protein-bound, drug is available for transfer. The amount of “free” drug delivered to the placenta depends on placental blood flow and the degree of maternal protein binding. The amount of drug available to the fetus depends on the degree of placental uptake, metabolism, and clearance (62). In single-dose drug studies, key factors influencing umbilical vein/maternal drug ratio are lipid solubility and transit time through the placental bed. In multidrug dosing (e.g., patient-controlled narcotic analgesia [PCA] delivery systems), key factors influencing fetal drug levels are the degree of ionization and degree of fetal protein binding (Fig. 16-3).
Fetal pH is lower than maternal pH; consequently, the fraction of opioid (and other basic drugs) existing in the ionized state is higher in the fetus than in the mother. Ionization results in drug trapping. The degree of ionization depends on the drug’s pKa; the effect is greater for meperidine (pKa ∼8.5) than morphine (pKa ∼8.0), and more significant when the fetus is acidotic. This is a simplistic, albeit true, application of opioid pharmacokinetics, a complex, difficult to predict, and incompletely evaluated topic.
All opioids have the potential to decrease baseline FHR and reduce variability, making interpretation of fetal CTG recordings potentially problematic. It has been documented from observational studies that parenteral narcotics can be associated with neonatal respiratory depression, decreased neonatal alertness, inhibition of sucking, and delay in effective feeding. When evidence related to the use of parenteral opioids for labor pain relief was subjected to a systematic review (63), it was noted that none of the studies was sufficiently powered to address the primary outcome measure of neonatal resuscitation, a measure of safety. Intramuscular opioid was compared to placebo, different i.m. opioid, same i.m. opioid but different dose, and same opioid given intravenously; IV opioid was compared to different IV opioid and same IV opioid but different modes of administration. There was insufficient pooled information to draw conclusions regarding any of the secondary outcome measures, including fetal distress administration of naloxone, Apgar score <7 at 5 minutes, baby death, admission to a special care setting, feeding problems, and problems with mother-baby interaction.
The concept of genetic imprinting at birth for opiate or amphetamine addiction in later life has been associated with systemically administered pain-relieving labor medications (narcotics, barbiturates, or nitrous oxide) (63). The original studies that led to this conclusion were criticized regarding the matching of controls and the imprinting hypothesis proposed to explain the finding (64). Although a more recent study of drug-abusing subjects confirmed the phenomenon, these results will be considered controversial until there is more confirmatory evidence.
Meperidine is the most commonly used opioid for labor analgesia worldwide. It has been shown that, as the time increases from administration of single-dose, i.m. meperidine 1.5 mg/kg during labor to delivery of the baby, so too does the level of meperidine in the fetus (65). Maximum fetal concentrations reach a plateau between 1 and 5 hours after dosing; therefore, babies born within 1 to
5 hours after meperidine is given to the mother have the greatest chance of narcotic-induced depression. In contrast to single-dose studies, multiple doses of meperidine administered over many hours lead to accumulation of meperidine’s metabolite, normeperidine, in the mother and fetus (66). Half-lives of 17-25 hours for this metabolite are common in the mother, whereas the half-life exceeds 60 hours in the fetus/newborn. Normeperidine is associated with respiratory depression, not reversible by naloxone, and seizures. Because of concerns about meperidine, research has focused on the newer, shorter-acting opioids with no active metabolites as alternatives.
5 hours after meperidine is given to the mother have the greatest chance of narcotic-induced depression. In contrast to single-dose studies, multiple doses of meperidine administered over many hours lead to accumulation of meperidine’s metabolite, normeperidine, in the mother and fetus (66). Half-lives of 17-25 hours for this metabolite are common in the mother, whereas the half-life exceeds 60 hours in the fetus/newborn. Normeperidine is associated with respiratory depression, not reversible by naloxone, and seizures. Because of concerns about meperidine, research has focused on the newer, shorter-acting opioids with no active metabolites as alternatives.
Fentanyl has been available clinically for more than 20 years. It offers prompt analgesia coupled with a short duration of action and no active metabolites. Both maternal and fetal drug levels decline in a parallel fashion following a single dose of the drug (67). In the first report of its administration to laboring patients, fentanyl (50 to 100 μg IV q1h) was compared with meperidine (25 to 50 mg IV q2-3hr) (68). More mothers were nauseated and sedated and more babies required naloxone in the meperidine group.
Sufentanil is the most lipid soluble (octanol:water partition coefficient 1778) of the commonly used opioids (61). This feature should enhance placental transfer after a single dose, but transfer is impeded by the extent of maternal plasma protein binding (α1-acid glycoprotein) and uptake by the placenta. Sufentanil concentration in the fetus rises slowly, reaching a plateau between 45 and 80 minutes postadministration (69). It is a useful maternal analgesic for pain relief during second stage, when fetal delivery is imminent (<45 minutes).
Remifentanil is a novel, ultra-short-acting opioid. It has the most rapid onset of peak effect (∼1 minute), shortest context-sensitive half time (∼3-5 minutes), and greatest clearance (40 mL/kg/min) of the commonly used opioids (61). Although the maternal cardiovascular and side effect profiles are similar to other fentanyl congeners, remifentanil is chemically distinct because of its ester linkages. This ester structure renders it susceptible to hydrolysis by red cell- and tissue-nonspecific esterases, resulting in rapid metabolism. Remifentanil concentration decreases by 50% within 3 to 5 minutes of stopping drug administration, regardless of the duration of the infusion (61).
Labor pain occurs at intervals and increases in intensity over time. Rapid recovery between contractions and after delivery is desirable. Therefore, the most effective means of administering remifentanil to the laboring patient, taking advantage of the drug’s characteristics, is via PCA with a background infusion. Bolus dose, lockout time, and rate of infusion can be titrated (70). The drug rapidly crosses the placenta and is quickly metabolized by fetal esterases (71). Maternal oxygen desaturation and sedation and reduced FHR variability have been observed (72). Newborns exposed to remifentanil in utero up until the time of delivery have been vigorous. There have been no reports of lower Apgar scores, unacceptable umbilical cord gas analyses, or respiratory depression necessitating naloxone use. (70,71,73)
Naloxone is used to reverse respiratory depression in narcotic-exposed newborns. It is contraindicated for infants of narcotic-dependent mothers, as administration may precipitate acute withdrawal and seizures. Naloxone has never been shown to reduce the need for assisted mechanical ventilation or reduce admission rates to special-care nurseries (74). No studies have evaluated the effect of naloxone on time to spontaneous effective respiration or long-term outcome. Naloxone use in adults has been associated with reports of hypertension, pulmonary edema, arrhythmias, and cardiac arrest (61).
Agonist-Antagonist Opioids
Nalbuphine is commonly used as a systemic analgesic during labor. Reports of severe perinatal cardiovascular and respiratory depression prompted Nicolle and associates (75) to carry out a study designed to delineate placental transfer and disposition of nalbuphine in the neonate. The estimated half-life was 4.1 hours (versus 0.9 hour in infants and 2 hours in adults). Given that the liver extensively metabolizes nalbuphine, the authors speculated that the slower neonatal plasma disappearance rate compared to the infant or adult could be due in part to immature hepatic function or bypass of the liver via the ductus venosus. All 28 babies had 5-minute Apgar scores of 10. Fifty-four percent of the FHR tracings showed reduced variability lasting 10-35 minutes after maternal injection.
One potential use for this class of drugs is in the treatment of opiate-dependent pregnant women. Babies born to mothers on a buprenorphine maintenance program showed little or no clinically measurable neonatal abstinence syndrome in contrast to findings with methadone, morphine, or heroin maintenance programs (76).
Nitrous Oxide
Nitrous oxide (N2O) is an odorless inhalational agent that exerts weak but prompt analgesic activity. It is a relatively insoluble gas at room temperature, and therefore equilibrates rapidly between the alveoli, blood, and brain. To be fully effective, inhalation needs to be timed with contractions such that the patient begins to breathe the gas about 10 to 15 seconds in advance of the next contraction. This synchronizes the peak effect of N2O with the zenith of pain, assuming the average contraction lasts 60 seconds and peaks at the midpoint. N2O is combined with oxygen in a 50:50 mixture for obstetric use and is self-administered by the patient through a specialized breathing circuit equipped with a demand valve. The negative pressure generated at the onset of inspiration opens the valve, which remains open during inspiration and closes when the patient begins to exhale. Inhalation analgesia with N2O during labor and delivery by itself, as a coanalgesic, or as a temporizing measure pending other forms of pain relief is less common in the United States than in other developed countries.
Any patient at risk of vitamin B12 deficiency (e.g., pernicious anemia or vegetarian) should not use N2O as it
irreversibly oxidizes vitamin B12, reducing the activity of methionine synthetase (necessary for myelin formation) and other B12-dependent enzymes. Many countries have set maximal environmental limits for N2O, which necessitates the use of ventilation systems that allow exhaled gas to be scavenged.
irreversibly oxidizes vitamin B12, reducing the activity of methionine synthetase (necessary for myelin formation) and other B12-dependent enzymes. Many countries have set maximal environmental limits for N2O, which necessitates the use of ventilation systems that allow exhaled gas to be scavenged.
N2O readily crosses the placenta. The maternal-fetal concentration ratio reaches 0.8 within 15 minutes of continuous inhalation. It has no effect on uterine contractions or FHR. It is not metabolized and is eliminated quickly and entirely by the lungs with the onset of respiration at birth. This is true whether the mother inhales N2O for 5 minutes or 5 hours. N2O does not affect Apgar scores or sucking behavior (77).
Paracervical Block
This peripheral block provides a therapeutic alternative for first-stage labor pain when central neuraxial blockade is contraindicated or unavailable. The technique involves transvaginal injection of LA on either side of the cervix to interrupt pain transmission at the level of the uterine and cervical plexuses (located at the base of the broad ligament). Paracervical block (PCB) is relatively easy to perform and, when effective, it provides good to excellent analgesia that lasts 1-2 hours.
Since the introduction of PCB in the 1940s, reports of serious adverse sequelae, including injection of LA directly into the uterine arteries or fetal head, fetal death, and profound bradycardia, have resulted in modification of the injection technique and changes to the concentration and type of LA used. There are statements cautioning against employing this block in situations of uteroplacental insufficiency or nonreassuring FHR tracings.
Overall, in current practice the incidence of fetal bradycardia postblock is about 15% (78), with the onset beginning 2 to 10 minutes after injection and the bradycardia lasting 15 to 30 minutes. The exact etiology is unknown; however, a recent investigation comparing epidural with PCB using Doppler flow velocity waveforms of the maternal femoral and uterine arteries, and umbilical and fetal middle cerebral arteries has shed some light on this phenomenon (79). PCB was associated with a small but significant increase in uterine artery impedance, indicating uterine artery vasoconstriction. In this study, Apgar scores and umbilical arterial and venous pH determinations were within the normal range in both groups.
Neuraxial Analgesia
Spinal, epidural, and combined spinal-epidural (CSE) techniques are commonplace for managing childbirth pain. They are used to administer opioids, LAs, and other pain-modulating adjuvants. Collectively, these methods are considered the most effective forms of pain relief available to laboring women.
Although spinal anesthesia has been in use since 1899, spinal analgesia only became a viable possibility in the 1970s following the discovery of specific opioid receptors in the brain and spinal cord. From a practical standpoint, however, it was not an option for laboring women at that time for two reasons: there was an unacceptably high incidence of postspinal headache (also known as postdural puncture headache) in the young female population; and a single injection technique could not be relied upon to provide analgesia for more than 1 to 2 hours. The advantage of having an epidural catheter in place, either for subsequent bolus dosing or for continuous infusion, is the provision of uninterrupted analgesia between placement of the catheter and delivery of the baby.
The (re-)introduction of fine-gauge, pencil-point, atraumatic (noncutting) spinal needles in the late 1980s fostered a renewed interest in subarachnoid (intrathecal) injection (80). With the advent of CSE equipment and “needle-through-needle” technique, single-level subarachnoid injection, followed immediately by epidural catheter placement at the same site, became possible (81). The CSE procedure has become synonymous with subarachnoid injection of opioid (± a small dose of LA) and simultaneous initiation of a low-dose epidural infusion. The perceived advantages of CSE compared with more traditional methods continue to be debated, along with the consequences of routine dural puncture (81,82,83).
Epidural catheter analgesia alone has been popular for many years. Depending on the choice of agents used, it provides superior pain relief during the first and second stages of labor and can be extended, if necessary, for cesarean section, instrumented vaginal delivery, manual placental removal, or episiotomy repair.
Neuraxial Opioids
Opioids injected into the lumbar intrathecal space distribute between nerve tissue and cerebrospinal fluid (CSF) on the basis of their partition coefficients (lipid solubility). Opioids injected into the epidural space first diffuse across the dura to reach the subarachnoid space, and then behave as their intrathecal counterparts. Morphine, the least lipid soluble of the commonly used opioids, diffuses slowly from the CSF into the substantia gelatinosa of the dorsal horn to activate opioid receptors. This accounts for its delayed onset and prolonged duration of action. Morphine also spreads rostrally, moving by bulk flow with CSF to reach vasomotor, respiratory, and vomiting centers in the brainstem. In contrast, the highly lipophilic fentanyl and sufentanil penetrate nerve tissue quickly. They have a faster onset of activity coupled with a shorter duration of action. Remifentanil is not approved for use in the intrathecal space because it contains a glycine preservative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree