Nutritional Support for The Pediatric Patient
Despite advances in the field of nutritional support, the prevalence of malnutrition among hospitalized patients, especially those with a protracted clinical course, has remained largely unchanged over the last two decades.1,2 The provision of optimal nutritional therapy requires a careful assessment of energy needs and the provision of macronutrients and micronutrients via the most suitable feeding route. The profound and stereotypic metabolic response to injury places unique demands on the hospitalized child. Standard equations available for estimating energy needs have proven to be unreliable in this population.3,4 In addition, children with critical illness have a marked net protein catabolism and often lack adequate nutritional support.5 Ultimately, an individualized nutritional regimen should be tailored for each child and reviewed regularly during the course of illness. An understanding of the metabolic events that accompany illness and surgery in a child is the first step in implementing appropriate nutritional support.
The Metabolic Response to Stress
The metabolic response to illness due to stressors such as trauma, surgery, or inflammation has been well described. Cuthbertson was the first investigator to realize the primary role that whole-body protein catabolism plays in the systemic response to injury.6 Based on his work, the metabolic stress response has been conceptually divided into two phases. The initial, brief ‘ebb phase’ is characterized by decreased enzymatic activity, reduced oxygen consumption, low cardiac output, and a core temperature that may be subnormal. This is followed by the hypermetabolic ‘flow phase’ characterized by increased cardiac output, oxygen consumption, and glucose production. During this phase, fat and protein mobilization is manifested by increased urinary nitrogen excretion and weight loss. This catabolic phase is mediated by a surge in cytokines and the characteristic endocrine response to trauma or operation that results in an increased availability of substrates essential for healing and glucose production.
Neonates and children share similar qualitative metabolic responses to illness as adults, albeit with significant quantitative differences. The metabolic stress response is beneficial in the short term, but the consequences of sustained catabolism are significant as the child has limited tissue stores and substantial nutrient requirements for growth. Thus the prompt institution of nutritional support is a priority in sick neonates and children. The goal of nutrition in this setting is to augment the short-term benefits of the metabolic response to injury while minimizing long-term consequences. In general, the metabolic stress response is characterized by an increase in net muscle protein degradation and the enhanced movement of free amino acids through the circulation (Fig. 2-1). These amino acids serve as the building blocks for the rapid synthesis of proteins that act as mediators for the inflammatory response and structural components for tissue repair. The remaining amino acids not used in this way are channeled through the liver where their carbon skeletons are utilized to create glucose through gluconeogenesis. The provision of additional dietary protein may slow the rate of net protein loss, but does not eliminate the overall negative protein balance associated with injury.7
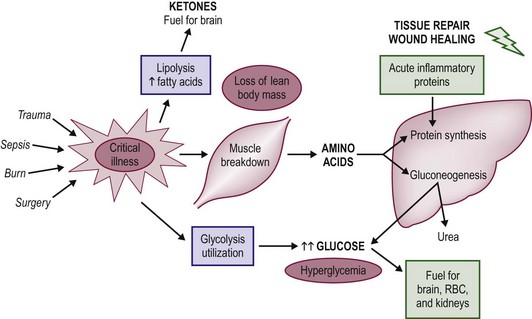
FIGURE 2-1 The metabolic changes associated with the pediatric stress response to critical illness and injury. In general, net protein catabolism predominates and amino acids are transported from muscle stores to the liver, where they are converted to inflammatory proteins and glucose through the process of gluconeogenesis.
Carbohydrate and lipid turnover are also increased several fold during the metabolic response. Although these metabolic alterations would be expected to increase overall energy requirements, data show that such an increase is quantitatively variable, modest, and evanescent. Overall, the energy needs of the critically ill or injured child are governed by the severity and persistence of the underlying illness or injury. Accurate assessment of energy requirements in individual patients allows optimal caloric supplementation and avoids the deleterious effects of both under- and overfeeding. Children with critical illness demonstrate a unique hormonal and cytokine profile characterized by an elevation in serum levels of insulin, the catabolic hormones (glucagons, cortisol, catecholamines), and specific cytokines known to interact with the inflammatory process.8 Novel ways to manipulate these hormonal and cytokine alterations with an aim to minimize the deleterious consequences induced by the stress response are a focus of research.
Body Composition and Nutrient Reserves
The body composition of the young child contrasts with that of the adult in several ways that significantly affect nutritional requirements. Table 2-1 lists the macronutrient stores of the neonate, child, and adult as a percentage of total body weight.9,10 Carbohydrate stores are limited in all age groups and provide only a short-term supply of glucose. Despite this fact, neonates have a high demand for glucose and have shown elevated rates of glucose turnover when compared with those of the adult.11 This is thought to be related to the neonate’s increased brain-to-body mass ratio because glucose is the primary energy source for the central nervous system. Neonatal glycogen stores are even more limited in the early postpartum period, especially in the preterm infant.12 Short periods of fasting can predispose the newborn to hypoglycemia. Thus when infants are burdened with illness or injury, they must rapidly turn to the breakdown of protein stores to generate glucose through the process of gluconeogenesis.
TABLE 2-1
The Body Composition of Neonates, Children, and Adults as a Percentage of Total Body Weight
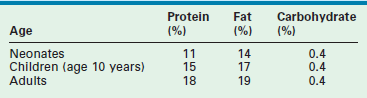
Lipid reserves are low in the neonate, gradually increasing with age. Premature infants have the lowest proportion of lipid stores as the majority of polyunsaturated fatty acids accumulate in the third trimester.13 This renders lipid less useful as a potential fuel source in the young child.14 The most dramatic difference between adult and pediatric patients is in the relative quantity of stored protein. The protein reserve of the adult is nearly twofold that of the neonate. Thus infants cannot afford to lose significant amounts of protein during the course of a protracted illness or injury. An important feature of the metabolic stress response, unlike in starvation, is that the provision of dietary glucose does not halt gluconeogenesis. Consequently, the catabolism of muscle protein to produce glucose continues unabated.15 Neonates and children also share much higher baseline energy requirements. Studies have demonstrated that the resting energy expenditure for neonates is two to three times that of adults when standardized for body weight.14,16 Clearly, the child’s need for rapid growth and development is a large component of this increase in energy requirement. Moreover, the relatively large body surface area of the young child may increase heat loss and further contributes to elevations in energy expenditure.
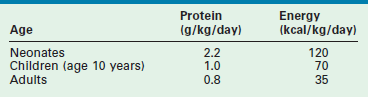
The basic requirements for protein and energy in the healthy neonate, child, and adult, based on recent recommendations by the National Academy of Sciences, are listed in Table 2-2.17 As illustrated, the recommended protein provision for the neonate is almost three times that of the adult. In premature infants, a minimum protein allotment of 2.8 g/kg/day is required to maintain in utero growth rates.18 The increased metabolic demand and limited nutrient reserves of the infant mandates early nutritional support in times of injury and critical illness to avoid negative nutritional consequences.
Accurate assessment of body composition is necessary for planning nutritional intake, monitoring dynamic changes in the body compartments (such as the loss of lean body mass), and assessing the adequacy of nutritional supportive regimens during critical illness. Ongoing loss of lean body mass is an indicator of inadequate dietary supplementation and may have clinical implications in the hospitalized child. However, current methods of body composition analysis (such as anthropometry, weight and biochemical parameters) are either impractical for clinical use or inaccurate in a subgroup of hospitalized children with critical illness. One of the principal problems in critically ill children is the presence of capillary leak, manifesting as edema and large fluid shifts. These make anthropometric measurements invalid and other bedside techniques have not been validated.
Energy Expenditure during Illness
REE can be measured using direct or indirect methods. The direct calorimetric method measures the heat released by a subject at rest and is based on the principle that all energy is eventually converted to heat. In practice, the patient is placed in a thermally isolated chamber, and the heat dissipated is measured for a given period of time.19 This method is the true gold standard for measured energy expenditure. Direct calorimetry is not practical for most hospitalized children and REE is often estimated using standard equations. Unfortunately, REE estimates using standardized World Health Organization (WHO) predictive equations are unreliable, particularly in underweight subjects.19–21
REE estimation is difficult in critically ill or postoperative children. Their energy requirements show individual variation and are dependent upon severity of injury. For instance, an infant with respiratory distress on pressure support is likely to have high energy requirement due to increased work of breathing. The same patient, when started on mechanical ventilation with muscle relaxants, is unlikely to have sustained high energy requirements. Infants with congenital diaphragmatic hernia on extracorporeal membrane oxygenation (ECMO) support have been shown to have energy expenditures of approximately 90 kcal/kg/day. Following extubation, the same patients may have energy requirements as high as 140 kcal/kg/day. Although stress factors ranging from 1.0 to 2.7 have been applied to correct for these variations, calculated standardized energy expenditure equations have not been satisfactorily validated in critically ill children.22–25
Indirect calorimetry measures VO2 (the volume of oxygen consumed) and VCO2 (the volume of CO2 produced), and uses a correlation factor based on urinary nitrogen excretion to calculate the overall rate of energy production.26 The measurement of energy needs is ‘indirect’ because it does not use direct temperature changes to determine energy needs. Indirect calorimetry provides a measurement of the overall respiratory quotient (RQ), defined as the ratio of CO2 produced to O2 consumed (VCO2/ VO2), for a given patient. Oxidation of carbohydrate yields an RQ of 1.0, whereas fatty acid oxidation gives an RQ of 0.7. However, the role of the RQ as a marker of substrate use and as an indicator of underfeeding or overfeeding is limited. The body’s ability to metabolize substrate may be impaired during illness, making assumptions invalid about RQ values and substrate oxidation.
Although RQ is not a sensitive marker for adequacy of feeding in individual cases, RQ values greater than 1.0 are generally associated with lipogenesis secondary to overfeeding.27,28 A recent study has suggested the utility of extremes of RQ in monitoring feeding adequacy, where an RQ higher than 0.85 reliably indicates the absence of underfeeding and an RQ higher than 1.0 reliably indicates the presence of overfeeding.29 However, numerous factors, related and unrelated to feeding, can alter the value of a measured RQ in critically ill patients, e.g., hyperventilation, acidosis, effects of cardiotonic agents and neuromuscular blocking, and an individual response to a given substrate load, injury, or disease. Furthermore, in the setting of wide diurnal and day-to-day variability of REE in critically ill individuals, the extrapolation of short-term calorimetric REE measurements to 24-hour REE may introduce errors. The use of steady-state measurements may decrease these errors. Steady state is defined by change in VO2 and VCO2 of <10% over a period of five consecutive minutes. The values for the mean REE from this steady-state period may be used as an accurate representation of the 24-hour TEE in patients with low levels of physical activity.30 In a patient who fails to achieve steady state and is metabolically unstable, prolonged testing is required (minimum of 60 minutes), and 24-hour indirect calorimetry should be considered. With the advent of newer technology, the application of indirect calorimetry at the bedside for continuous monitoring shows promise.
Indirect calorimetry is not accurate in the setting of air leaks around the endotracheal tube, ventilator circuit or through a chest tube, or in subjects on ECMO. High inspired oxygen fraction (FiO2 >0.6) will also affect indirect calorimetry. Indirect calorimetry is difficult to use in babies on ECMO because a large proportion of the patient’s oxygenation and ventilation is performed through the membrane oxygenator. The use of indirect calorimetry for assessment and monitoring of nutrition intake requires attention to its limitations and expertise in the interpretation. Nonetheless, its application in children at high risk for underfeeding and overfeeding may be helpful.31,32
Nonradioactive stable isotope techniques have been used to measure REE in the pediatric patient. Stable isotope technology has been available for many years and was first applied for energy expenditure measurement in humans in 1982.33,34 Both 13C-labeled bicarbonate and doubly labeled water (2H218O) have been used to measure TEE in pediatric surgical patients, and have been shown to correlate well with indirect calorimetry.31,34,35 The 13C-labeled bicarbonate method allows the calculation of REE on the basis of infusion rate and the ratio of labeled to unlabeled CO2 in expired breath samples.35 Orally administered stable isotopes of water (2H2O and H218O) mix with the body water and the 18O is lost from the body as both water and CO2, while the 2H is lost from the body only as water. The difference in the rates of loss of the isotopes 18O and 2H from the body reflects the rate of CO2 production, which can be used to calculate the TEE.36–38 However, the doubly labeled water method has its limitations in children with active capillary leak, decreased urine output, fluid overload, and diuretic use.36
In general, any increase in energy expenditure during illness or after an operation is variable, and studies suggest that the increase is far less than originally hypothesized. In children with severe burns, the initial REE during the flow phase of injury is increased by 50% but then returns to normal during convalescence.39 In neonates with bronchopulmonary dysplasia, in which the illness increases the patient’s work of breathing, a 25% elevation in energy requirement is evident.40 Newborns undergoing major surgery have only a transient 20% increase in energy expenditure that returns to baseline values within 12 hours postoperatively, provided no major complications develop.41,42 Stable extubated neonates, five days after operation, have been shown to have REE comparable to normal infants.43 Effective anesthetic and analgesic management may play a significant role in muting the stress response of the neonate. Studies have demonstrated no discernible increase in REE in neonates undergoing patent ductus arteriosus ligation who received intraoperative fentanyl anesthesia and postoperative intravenous analgesic regimens.42 A retrospective stratification of surgical infants into low- and high-stress cohorts based on the severity of underlying illness found that high-stress infants undergo moderate short-term elevations in energy expenditure after operation, whereas low-stress infants do not manifest any increase in energy expenditures during the course of illness.44 Finally, by using stable isotopic methods, it has been found that the mean energy expenditures of critically ill neonates on ECMO are nearly identical to age- and diet-matched nonstressed controls.45
All these studies suggest that critically ill neonates have only a small and usually short-term increase in energy expenditure. Although children have increased energy requirements from increased metabolic turnover during illness, their caloric needs may be lower than previously considered due to possible halted or slow growth,46 and the use of sedation and muscle paralysis.47 This could result in overfeeding when energy intake is based on presumed or estimated energy expenditure with stress factors. On the other hand, unrecognized hypermetabolism in select individuals result in underfeeding with negative nutritional consequences.31 The variability in energy requirements may result in cumulative energy imbalances in the intensive care unit (ICU) over a period of time.32 A direct relationship has been reported between cumulative caloric imbalance and the mortality rate in critically ill surgical patients.48
For practical purposes, the recommended dietary caloric intake for healthy children may represent a reasonable starting point for the upper limit of caloric allotment in the hospitalized child.17 However, as discussed earlier, energy requirement estimates in select groups of patients remain variable and possibly overestimated, mandating an accurate estimation using measured energy expenditure where available. Regular anthropometric measurements plotted on a growth chart to assess the adequacy of caloric provision will allow relatively prompt detection of underfeeding or overfeeding in most cases. However, some critically ill children may be too sick for regular weights or have changes in body water that make anthropometric measurements unreliable.
Macronutrient Intake
Protein Metabolism and Requirement During Illness
Amino acids are the key building blocks required for growth and tissue repair. The vast majority (98%) are found in existing proteins, and the remainder reside in the free amino acid pool. Proteins are continually degraded into their constituent amino acids and resynthesized through the process of protein turnover. The reutilization of amino acids released by protein breakdown is extensive. Synthesis of proteins from the recycling of amino acids is more than two times greater than from dietary protein intake. An advantage of high protein turnover is that a continuous flow of amino acids is available for the synthesis of new proteins. This allows the body tremendous flexibility in meeting ever-changing physiologic needs. However, the process of protein turnover requires the input of energy to power both protein degradation and synthesis. At baseline, infants are known to have higher rates of protein turnover than adults. Healthy newborns have a protein-turnover rate of 6–12 g/kg/day compared with 3.5 g/kg/day in adults.49 Even greater rates of protein turnover have been measured in premature and low birth weight infants.50 For example, it has been demonstrated that extremely low birth weight infants receiving no dietary protein can lose in excess of 1.2 g/kg/day of endogenous protein.51 At the same time, infants must maintain a positive protein balance to attain normal growth and development, whereas the healthy adult can subsist with a neutral protein balance.
In the metabolically stressed patient, such as the child with severe burn injury or cardiorespiratory failure requiring ECMO, protein turnover is doubled when compared with normal subjects.34,49 A study of critically ill infants and children found an 80% increase in protein turnover, which correlated with the duration of the critical illness.52 This process redistributes amino acids from skeletal muscle to the liver, wound, and tissues taking part in the inflammatory response. The factors required for the inflammatory response (acutely needed enzymes, serum proteins, and glucose) are thereby synthesized from degraded body protein stores. The well-established increase in hepatically derived acute phase proteins (including C-reactive protein, fibrinogen, transferrin, and α-1-acid glycoprotein), along with the concomitant decrease in transport proteins (albumin and retinol-binding protein), is evidence of this protein redistribution. As substrate turnover is increased during the stress response, rates of both whole-body protein degradation and whole-body protein synthesis are accelerated. However, protein breakdown predominates, thereby leading to a hypercatabolic state with an ensuing net negative protein and nitrogen balance.27
Protein loss is evident in elevated levels of excreted urinary nitrogen during critical illness. For example, infants with sepsis demonstrate a severalfold increase in the loss of urinary nitrogen that directly correlates with the degree of illness.53 Clinically, severe protein loss can be manifested by skeletal muscle wasting, weight loss, delayed wound healing, and immune dysfunction.54 In addition to the reprioritization of protein for tissue repair, healing and inflammation, the body appears to have an increased need for glucose production during times of metabolic stress.55 The accelerated rate of gluconeogenesis during illness and injury is seen in both children and adults, and this process appears to be accentuated in infants with low body weight.13,54 The increased production of glucose in times of illness is necessary as glucose represents a versatile energy source for tissues taking part in the inflammatory response. For example, it has been shown that glucose utilization by leukocytes is significantly increased during the inflammatory response.56 Unfortunately, the provision of additional dietary glucose does not suppress the body’s need for increased glucose production. Therefore, net protein breakdown continues to predominate.14,57,58
Specific amino acids are transported from muscle to the liver to facilitate hepatic glucose production. The initial step of amino acid catabolism involves removal of the toxic amino group (NH3). Through transamination, the amino group is transferred to α-ketoglutarate, thereby producing glutamate. The addition of another amino group converts glutamate to glutamine, which is subsequently transported to the liver. Here, the amino groups are removed from glutamine and detoxified to urea through the urea cycle. The amino acid carbon skeleton can then enter the gluconeogenesis pathway. Alternatively, in skeletal muscle, the amino group can be transferred to pyruvate, thereby forming alanine. When alanine is transported to the liver and detoxified, pyruvate is reformed and can be converted to glucose through gluconeogenesis. The transport of alanine and pyruvate between peripheral muscle tissue and the liver is termed the glucose-alanine cycle.59 Hence the transport amino acid systems involving glutamine and alanine provide carbon backbones for gluconeogenesis, while facilitating the hepatic detoxification of ammonia by the urea cycle.
Increased muscle protein catabolism is a successful short-term adaptation during critical illness, but it is limited and ultimately harmful to the child with reduced protein stores and elevated protein demands. Unless the inciting stress is eliminated, the progressive breakdown of diaphragmatic, cardiac, and skeletal muscle can lead to respiratory compromise, fatal arrhythmia, and loss of lean body mass. Moreover, a prolonged negative protein balance may have a significant impact on the child’s growth and development. Healthy, nonstressed neonates require a positive protein balance of nearly 2 g/kg/day.48,60 In contrast, critically ill, premature neonates requiring mechanical ventilation have a negative protein balance of −1 g/kg/day.61,62 Critically ill neonates who require ECMO have exceedingly high rates of protein loss, with a net negative protein balance of –2.3 g/kg/day.63 It has been well established that the extent of protein catabolism correlates with morbidity and mortality in surgical patients.
Fortunately, amino acid supplementation tends to promote increased nitrogen retention and positive protein balance in critically ill patients.59,64 The mechanism appears to be an increase in protein synthesis while rates of protein degradation remain constant.60,61 Therefore the provision of dietary protein sufficient to optimize protein synthesis, facilitate wound healing and the inflammatory process, and preserve skeletal muscle mass is the single most important nutritional intervention in critically ill children. The quantity of protein needed to enhance protein accrual is greater in hospitalized sick children than in healthy children. Table 2-3 lists recommended quantities of dietary protein for hospitalized children. Extreme cases of physiologic stress, including the child with extensive burns or the neonate on ECMO, may necessitate additional protein supplementation to meet metabolic demands.
TABLE 2-3
Recommended Protein Requirements for Hospitalized Infants and Children
| Age (years) | Estimated Protein Requirement (g/kg/day) |
| Extremely low birth weight infants | up to 3.5 |
| Very low birth weight | up to 3.0 |
| 0–2 | 2.0–3.0 |
| 2–13 | 1.5–2.0 |
| 13–18 | 1.0–1.5 |
The influence of macronutrient intake on protein balance has been explored in a limited number of studies. A systematic review of all such studies in mechanically ventilated children showed that a minimum of 1.5 g/kg/day protein and 57 kcal/kg/day energy intake was needed to achieve a positive protein balance in this group.62 However, it should be noted that toxicity from excessive protein administration can occur, particularly in children with impaired renal and hepatic function. The provision of protein at levels greater than 3 g/kg/day is rarely indicated and is often associated with azotemia. In premature neonates, the possible beneficial effects of protein allotments of 3–3.5 g/kg/day are being actively investigated in an effort to replicate intrauterine growth rates. Studies using protein provisions of 6 g/kg/day in children have demonstrated significant morbidity, including azotemia, pyrexia, strabismus, and lower IQ scores.64,65
Protein Quality
In addition to the sufficient quantity of dietary protein, an increased focus has been placed on the protein quality of nutritional provisions. The specific amino acid formulation to best increase whole-body protein balance has yet to be fully determined, although numerous clinical and basic science research projects are actively focusing on this topic. It is known that infants have an increased requirement per kilogram for the essential amino acids compared to the adult.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree