Fetal body composition changes throughout gestation, with accretion of most nutrients occurring primarily in the late second and throughout the third trimester. Term infants will normally have sufficient glycogen and fat stores to meet energy requirements during the relative starvation of the first day after birth. In contrast, preterm infants will rapidly deplete their limited nutrient reserves, becoming both hypoglycemic and catabolic unless appropriate nutritional therapy is provided. In practice, it is generally assumed that the severity of nutrient insufficiency is inversely related to gestational age at birth and birth weight.
Postnatal growth varies from intrauterine growth in that it begins with a period of weight loss, primarily through the loss of extracellular fluid. The typical loss of 5% to 10% of birth weight for a full-term infant may increase to as much as 15% of birth weight in infants born preterm. The nadir in weight loss usually occurs by 4 to 6 days of life, with birth weight being regained by 14 to 21 days of life in most preterm infants. Currently, there is no widely accepted measure of neonatal growth that captures both the weight loss and subsequent gain characteristic of this period. Goals in practice are to limit the degree and duration of initial weight loss in preterm infants and to facilitate regain of birth weight within 7 to 14 days of life.
After achieving birth weight, intrauterine growth and nutrient accretion rate data are widely accepted as reference standards for assessing growth and nutrient requirements. Goals of 10 to 20 g/kg/day weight gain (15—20 g/kg/day for infants < 1,500 g), approximately 1 cm/week in length, and 0.5 to 1 cm/week in head circumference are used. Although these goals are not initially attainable in most preterm infants, replicating growth of the fetus at the same gestational age remains an appropriate goal as recommended by the American Academy of Pediatrics (AAP).
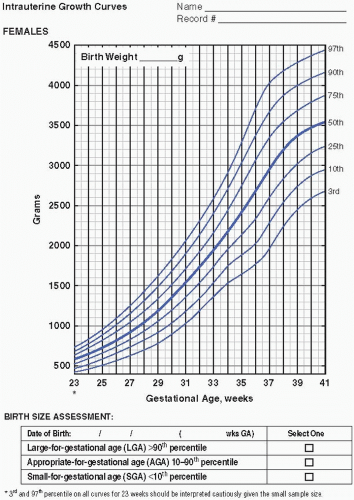
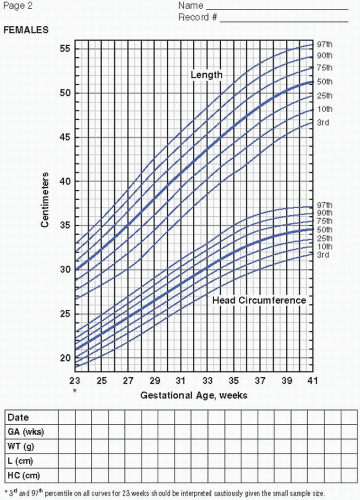
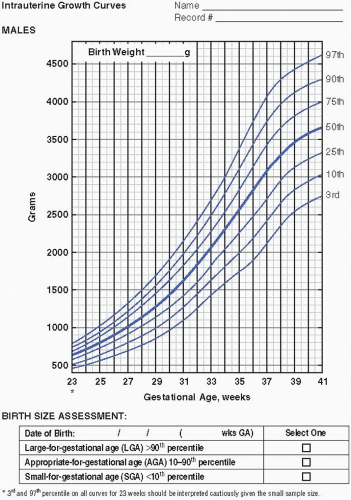
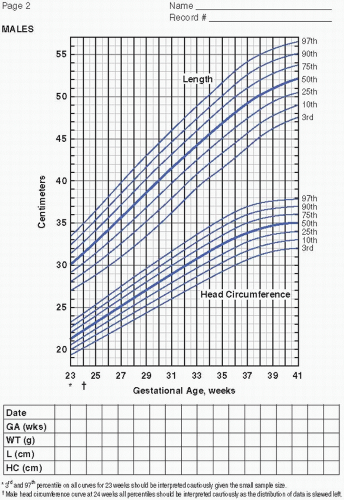
Serial measurements of weight, head circumference, and length plotted on growth curves provide valuable information in the nutritional assessment of the preterm infant. Historically, the Lubchenco intrauterine growth curves (1966) have been widely used because the chart is based on a reasonable sample size, provides curves
to monitor weight, length, and head circumference, and is easy to use and interpret. Of late, the Fenton (2003) fetal-infant chart has been more frequently utilized. The chart is based on a larger number of infants from a wider geographic location and reflects infants born more recently. With the Fenton chart, the premature infant’s growth can be monitored for a longer period of time, from 22 to 50 weeks postmenstrual age (PMA). Most recently, the Olsen (2010) growth curves have become available (Figure 21.1A—D). These newer growth charts are drawn from a large, contemporary, racially diverse US sample. Gender-specific weight, length, and head circumference curves are provided. Postnatal growth curves are also available. Postnatal growth curves follow the same infants over time (i.e., longitudinal growth curves), and are available from a number of single—neonatal intensive care unit (NICU) studies and from the National Institute for Child Health and Human Development (NICHD) multicenter study (2000). These curves, however, show actual, not ideal, growth. Although these curves provide interesting information by allowing comparison of the growth of infants in one NICU to those in another, they do not indicate if either group of infants is growing adequately. Intrauterine growth remains the gold standard for comparison.
When an infant is in full-term corrected gestational age, the Centers for Disease Control and Prevention (CDC) recommends the World Health Organization (WHO) Child Growth Standards 2006 be used for monitoring of growth. Infants should be plotted by corrected age and followed for catch-up growth. The charts can be downloaded from www.cdc.gov/growthcharts/who_charts.htm.
Sources for nutrient recommendations for preterm infants include the American Academy of Pediatrics Committee on Nutrition (AAP-CON), the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Committee on Nutrition (ESPGHAN-CON), and the Reasonable Ranges of Nutrient Intakes published by Tsang and colleagues (Table 21.1). These recommendations are based on (i) intrauterine accretion rate data, (ii) the nutrient content of human milk, (iii) the assumed decreased nutrient stores and higher nutritional needs in preterm infants, and (iv) the available data on biochemical measures reflecting adequate intake. However, due to the limitations of the currently available data, the goals for nutrient intake for preterm infants are considered to be recommendations only.
Fluid (see Chaps. 13 and 23). The initial step in nutritional support is to determine an infant’s fluid requirement, which is dependent on gestational age, postnatal age, and environmental conditions. Generally, baseline fluid needs are inversely related to gestational age at birth and birth weight. During the first week of life, very low birth weight (VLBW) infants are known to experience increased water loss because of the immaturity of their skin, which has a higher water content and increased permeability, and the immaturity of their renal function with a decreased ability to concentrate urine. Environmental factors, such as radiant warmers and phototherapy versus humidified incubators, also impact insensible losses and may affect fluid requirements. Conversely, restriction of fluid intake may be necessary to assist with the prevention and/or treatment of patent ductus arteriosus, renal insufficiency, and bronchopulmonary dysplasia (BPD). Fluid requirements in the first weeks of life are, therefore, continually reassessed, as the transition is made from fetal to neonatal life and at least daily afterward.
Table 21.1 Comparison of Enteral Intake Recommendations and Selected Feeding Regimens* for the Preterm Infant
Nutrient
Unit
Enteral intake recommendations for growing, stable preterm infants (2, 8)
Mature human milk‡
Mature human milk plus 4 packets Enfamil HMF/dL
Mature human milk plus 4 vials Enfamil HMF acidified liquid
Mature human milk plus 4 packets Similac HMF/dL
Mature human milk plus Prolact +4
24 kcal/oz Enfamil Premature w/Iron
24 kcal/oz Similac Special Care w/Iron
Protein‡‡
g/kg/day
1.6
3.2
4
3
3
3.6
3.6
ELBW infants
g/kg/day
3.8-4.4
VLBW infants
g/kg/day
3.4-4.2
Carbohydrate
g/kg/day
10.8
11.4
9.7
13.5
11.4
13.4
12.6
ELBW infants
g/kg/day
9-20
VLBW infants
g/kg/day
7-17
Fat
g/kg/day
5.9
7.4
7.7
6.4
7.4
6.2
6.6
ELBW infants
g/kg/day
6.2-8.4
VLBW infants
g/kg/day
5.3-7.2
Docosahexaenoic acid
mg/kg/day
≥15
20.7
17.1
ELBW infants
mg/kg/day
≥21
VLBW infants
mg/kg/day
≥18
Arachidonic acid
mg/kg/day
42
26.9
ELBW infants
mg/kg/day
≥28
VLBW infants
mg/kg/day
≥24
Vitamin A
IU/kg/day
700-1,500
338
1,763
1,731
1,268
360
1,515
1,521
Vitamin D
IU/day
150-400‡‡‡
3
228
237
183
42
292.5
183
Vitamin E
IU/kg/day
6-12
0.6
7.5
7.5
5.4
1
7.7
4.8
Vitamin K
mcg/kg/day
8-10
0.3
6.9
7.4
12.8
0.2
9.8
14.6
Thiamine
mcg/kg/day
180-240
32
257
257
381
31.5
243
304.5
Riboflavin
mcg/kg/day
250-360
52
382
373
677
64.5
360
754.5
Vitamin B6
mcg/kg/day
150-210
30.6
203
200
347
24
183
304.5
Vitamin B12
mcg/kg/day
0.3
0.07
0.3
1
1
0.06
0.3
0.67
Niacin
mg/kg/day
3.6-4.8
0.2
4.7
4.8
5.6
0.26
4.8
6.1
Folate
mcg/kg/day
25-50
7.2
44.7
44.4
41.7
13.5
48
45
Pantothenic acid
mg/kg/day
1.2-1.7
0.27
1.4
1.4
2.5
0.33
1.5
2.3
Biotin
mcg/kg/day
3.6-6
0.6
4.7
4.7
39.6
0.75
4.8
45
Vitamin C
mg/kg/day
18-24
6.1
24
24
43.5
4.5
24.3
45
Choline
mg/kg/day
14.4-28
14.3
17.2
12
24.3
12
Inositol
mg/kg/day
32-81
22.5
28.5
18
54
48
Taurine
mg/kg/day
4.5-9
7.3
Carnitine
mg/kg/day
˜2.9
2.9
Calcium
mg/kg/day
100-220
42
177
180
217.5
210
201
219
Phosphorus
mg/kg/day
60-140
21.4
96.3
97
121.9
121.5
100.5
121.5
Magnesium
mg/kg/day
7.9-15
5.2
6.7
6.6
15.6
11.9
11
14.6
Iron
mg/kg/day
2-4
0.04
2.2
2.2
0.6
0.15
2.2
2.2
Zinc
mcg/kg/day
1,000-3,000
183
1,263
1,352
1,683
960
1,830
1,830
Manganese
mcg/kg/day
0.7-7.5
1
16
13
11.8
19.5
7.7
15
Copper
mcg/kg/day
120-150
37.8
103.8
107
292.8
121.5
145.5
304.5
Iodine
mcg/kg/day
10-60
16.3
13.5
30
7.5
Selenium
mcg/kg/day
1.3-4.5
2.3
3
3.5
2.3
Sodium
mEq/kg/day
3-5
1.2
2.2
2.5
2.2
3.3
3.1
2.3
Potassium
mEq/kg/day
2-3
2
3.1
3.1
4.4
3
3.1
4
Chloride
mEq/kg/day
3-7
1.8
2.3
2.5
3.4
3
3.1
2.9
HMF = Human Milk Fortifier
*Calculated intakes of human milk feedings and formulas are based on an intake of 150 mL/kg/day.
‡ ‡ Denotes milk of mothers of preterm infants post the first 21 days of lactation.
‡‡ ‡‡ The AAP suggests the estimated requirements based on the fetal accretion rate of protein are 3.5 to 4 g/kg/day.
‡‡‡ ‡‡‡ Aim for 400 IU/day.
Energy. Estimates suggest that preterm infants in a thermoneutral environment require approximately 40 to 60 kcal/kg/day for maintenance of body weight, assuming adequate protein is provided. Additional calories are needed for growth, with the smallest neonates tending to demonstrate the greatest need, as their rate of growth is highest (Table 21.2). The AAP recommends 105 to 130 kcal/kg/day. Practice generally strives for energy intakes of 110 to 130 kcal/kg/day. Infants with severe and/or prolonged illness frequently require a range of 130 to 150 kcal/kg/day. Lesser intakes (90—120 kcal/kg/day) may sustain intrauterine growth rates if energy expenditure is minimal or if parenteral nutrition (PN) is used.
Nutrient goals. Our initial goal for PN is to provide adequate calories and amino acids to prevent negative energy and nitrogen balance. Goals thereafter include the promotion of appropriate weight gain and growth, while awaiting the attainment of adequate enteral intake.
Indications for initiating PN
PN is started the first postnatal day for infants who are <1,500 g birth weight. Infants for whom significant enteral intake is not anticipated by 5 days of age or those with cardiac disease requiring calcium supplementation may also be considered for PN.
Table 21.2 Estimation of Energy Requirement of the Low Birth Weight Infant*
Average estimation, kcal/kg/day
Energy expended
40-60
Resting metabolic rate
40-50†
Activity
0-5†
Thermoregulation
0-5†
Synthesis
15‡
Energy stored
20-30‡
Energy excreted
15
Energy intake
90-120
*From: American Academy of Pediatrics, Committee on Nutrition (AAP-CON). Pediatric Nutrition Handbook. 6th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009.
† †Energy for maintenance.
‡ ‡Energy cost of growth.
Peripheral versus central PN
Parenteral solutions may be infused through peripheral veins or a central vein, accessed from the antecubital and saphenous veins, respectively. Historically, the AAP has recommended that peripheral solutions maintain an osmolarity between 300 and 900 mOsm/L. Because of this limitation, peripheral solutions often cannot adequately support growth in extremely low birth weight (ELBW) infants. Central PN not only allows for the use of more hypertonic solutions but also incurs greater risks, particularly catheter-related sepsis.
Central PN is considered to be warranted under the following conditions:
Nutritional needs exceed the capabilities of peripheral PN
An extended period (e.g., >7 days) of inability to take enteral feedings, such as in infants with necrotizing enterocolitis (NEC) and in some postoperative infants
Imminent lack of peripheral venous access
Carbohydrate. Dextrose (D-glucose) is the carbohydrate source in intravenous (IV) solutions (see Chap. 24).
The caloric value of dextrose is 3.4 kcal/g.
Because dextrose contributes to the osmolarity of a solution, it is generally recommended that the concentration administered through peripheral veins be limited to ≤12.5% dextrose. Higher concentrations of dextrose may be used for central venous infusions in circumstances when fluid volume is severely restricted. Infants receiving extracorporeal membrane oxygenation (ECMO) therapy may require up to 40% dextrose.
Dextrose infusions are typically referred to in terms of the milligrams of glucose per kilogram per minute (mg/kg/min) delivered, which expresses the total glucose load and accounts for infusion rate, dextrose concentration, and patient weight (Figure 21.2).
The initial glucose requirement for term infants is defined as the amount that is necessary to avoid hypoglycemia. In general, this may be achieved with initial infusion rates of approximately 4 mg/kg/minute.
Preterm infants usually require higher rates of glucose, as they have a higher brain-to-body weight ratio and higher total energy needs. Initial infusion rates of 4 to 8 mg/kg/minute may be required to maintain euglycemia.
Initial rates may be advanced, as tolerated, by 1 to 2 mg/kg/minute daily to a maximum of 11 to 12 mg/kg/minute. This may be accomplished by increasing dextrose concentration, by increasing infusion rate, or by a combination of both. Infusion rates above 11 to 12 mg/kg/minute may exceed the infant’s oxidative capacity and are generally not recommended, as this may cause the excess glucose to be converted to fat, particularly in the liver. This conversion may also increase oxygen consumption, energy expenditure, and CO2 production.
The quantity of dextrose that an infant can tolerate will vary with gestational and postnatal age. Signs of glucose intolerance include hyperglycemia and secondary glucosuria with osmotic diuresis.
Protein. Crystalline amino acid solutions provide the nitrogen source in PN.
The caloric value of amino acids is 4 kcal/g.
Three pediatric amino acid formulations are commercially available in the United States: TrophAmine (B. Braun), Aminosyn-PF (Hospira), and PremaSol
(Baxter). In theory, these products are better adapted to the needs of newborns than are standard adult formulations, as they have been modified for improved tolerance and contain conditionally essential amino acids. However, the optimal amino acid composition for neonatal PN has not yet been defined, and there are no products currently available that are specifically designed for preterm infants.
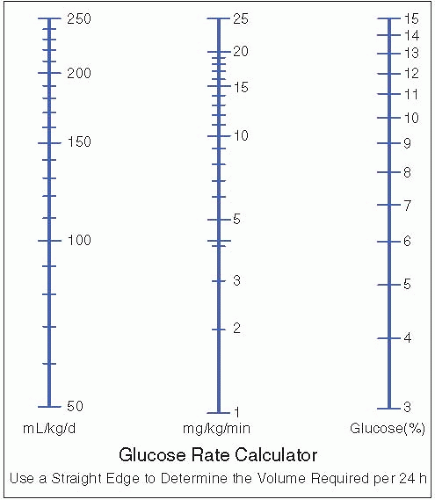
Figure 21.2. Interconversion of glucose infusion units. From Klaus MH, Faranoff AA, eds. Care of the High-Risk Neonate. 2nd ed. Philadelphia: WB Saunders, 1979:430.
It has been demonstrated that VLBW infants who do not receive amino acids in the first postnatal days catabolize body protein at a rate of at least 1 g/kg/day. Studies investigating the use of early amino acids have consistently shown a reversal of this catabolism without adverse metabolic consequences. Current recommendations support the infusion of amino acids in a dose of 2 g/kg/day beginning in the first 24 hours after birth.
Infants with a birth weight <1,250 g are provided with 2 to 2.4 g/kg/day beginning immediately after birth. Infants with a birth weight between 1,250 and 1,500 g are initiated on 2 g/kg/day within the first 24 hours of life. Infants >1,500 g are only initiated on 2 g/kg/day if indicated, depending on their size, clinical condition, and estimated time to achieve significant enteral volumes.
Protein infusion rates are generally advanced to a target of 3.5 g/kg/day for premature infants and up to 3 g/kg/day for the term neonates.
Lipid. Soybean oil, or a combination of soybean and safflower oil, provides the fat source for IV fat emulsions.
The caloric value of 20% lipid emulsions is 2 kcal/mL (approximately 10 kcal/g). The use of 20% emulsions is preferred over 10% because the higher ratio of phospholipids to triglyceride in the 10% emulsion interferes with plasma triglyceride clearance. Twenty percent emulsions also provide a more concentrated source of calories. For these reasons, only 20% lipid emulsions are used.
Current data suggest that preterm infants are at risk for essential fatty acid (EFA) deficiency within 72 hours after birth if an exogenous fat source is not delivered. This deficiency state can be avoided by the administration of 0.5 to 1 g/kg/day of lipid emulsion. Therefore, infants weighing < 1,500 g at birth are provided with approximately 1 to 2 g/kg/day within the first 24 to 48 hours after birth. This rate is advanced by approximately 1 g/kg/day, as tolerated, to a target of 3 g/kg/day.
Tolerance also correlates with hourly infusion rate, and no benefit to a rest period has been identified. Therefore, lipid emulsions are infused over 24 hours for optimal clearance. However, due to sepsis risk factors, syringes may be changed every 12 hours.
Electrolytes
Sodium and potassium concentrations are adjusted daily based on individual requirements (see Chap. 23). Maintenance requirements are estimated at approximately 2 to 4 mEq/kg.
Increasing the proportion of anions provided as acetate aids in the treatment of metabolic acidosis in VLBW infants.
Vitamins. The current vitamin formulations (MVI Pediatric, Hospira, INFUVITE Pediatric, Baxter) do not maintain blood levels of all vitamins within an acceptable range for preterm infants. However, there are no products currently available that are specifically designed for preterm infants. Table 21.3 provides guidelines for the use of the available formulations for term and preterm infants. For infants less than 2,500 g, the AAP suggests a dose of 40% of the MVI Pediatric (INFUVITE Pediatric) 5 mL vial/kg/day. This guideline may be met by adding 1.5 mL MVI Pediatric/100 mL PN and administered at a rate of approximately 140 mL/kg. For infants 2,500 g or greater, the AAP suggests the 5 mL MVI Pediatric (INFUVITE Pediatric) per day. Vitamin A is the most difficult to provide in adequate amounts to the VLBW infant without providing excess amounts of the other vitamins, as it is subject to losses through photodegradation and absorption to plastic tubing and solution-containing bags. B vitamins may also be affected by photodegradation. This is of particular concern with long-term PN use and, for this reason, consideration should be given to shielding the PN-containing plastic bags and tubing from light.
Minerals. The amount of calcium and phosphorus that can be administered through IV is limited by the precipitation of calcium phosphate. Unfortunately, the variables that determine calcium and phosphate compatibility in PN are complex and what constitutes maximal safe concentrations is controversial. The aluminum content of these preparations should also be considered.
Table 21.3 Suggested Intakes of Parenteral Vitamins in Infants
Vitamins
Estimated needs
2 mL of a 5 mL singledose vial MVI Pediatric (Hospira), INFUVITE Pediatric (Baxter)
1.5 mL MVI Pediatric (Hospira), INFUVITE Pediatric (Baxter) per 100 mL PN administered at a rate of 140 mL/kg/day†
Term infants (≥2.5 kg) (dose/day)
Preterm infants (≤2.5 kg)*
Lipid soluble

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access