Chapter 209 Nontuberculous Mycobacteria
Clinical Manifestations
Lymphadenitis of the superior anterior cervical or submandibular lymph nodes is the most common manifestation of NTM infection in children (Table 209-1). Preauricular, posterior cervical, axillary, and inguinal nodes are involved occasionally. Lymphadenitis is most common in children 1-5 yr of age and has been related to their tendency to put objects contaminated with soil, dust, or standing water into their mouths. Given the constant environmental exposure to NTM, the occurrence of these infections might also reflect an atypical immune response of a subset of the infected children during or after their first contact with NTM.
Table 209-1 DISEASES CAUSED BY NONTUBERCULOUS MYCOBACTERIAL SPECIES
| CLINICAL DISEASE | COMMON SPECIES | LESS-COMMON SPECIES |
|---|---|---|
| Cutaneous infection | M. chelonae, M. fortuitum, M. abscessus, M. marinum | M. ulcerans* |
| Lymphadenitis | MAC | M. kansasii, M. haemophilum, M. malmoense† |
| Otologic infection | M. abscessus, MAC | M. fortuitum |
| Pulmonary infection | MAC, M. kansasii, M. abscessus | M. xenopi, M. malmoense,† M. szulgai, M. fortuitum, M. simiae |
| Catheter-associated infection | M. chelonae, M. fortuitum | M. abscessus |
| Skeletal infection | MAC, M. kansasii, M. fortuitum | M. chelonae, M. marinum, M. abscessus, M. ulcerans* |
| Disseminated | MAC | M. kansasii, M. genavense, M. haemophilum, M. chelonae |
MAC, Mycobacterium avium complex.
† Found primarily in Northern Europe.
From American Academy of Pediatrics: Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, p 703.
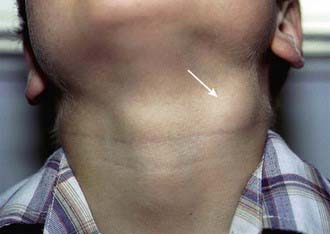
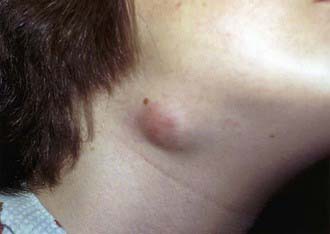
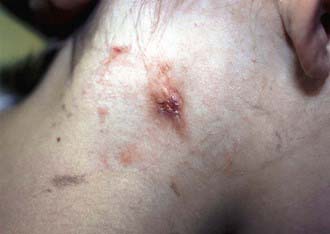
Affected children usually lack constitutional symptoms and present with a unilateral subacute and slowly enlarging lymph node or group of closely approximated nodes >1.5 cm that are firm, painless, freely movable, and not erythematous (Fig. 209-1). The involved nodes occasionally resolve without treatment, but most undergo rapid suppuration after several weeks (Fig. 209-2). The center of the node becomes fluctuant, and the overlying skin becomes erythematous and thin. Eventually, the nodes rupture and form cutaneous sinus tracts that drain for months or years, resembling the classic scrofula of tuberculosis (Fig. 209-3).
Cutaneous disease caused by NTM is rare in children (see Table 209-1). Infection usually follows percutaneous inoculation with fresh or salt water contaminated by M. marinum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree