Materials and Methods
Objective
The aim of this systematic review and meta-analysis of randomized clinical trials was to evaluate the effectiveness of neuraxial analgesia as intervention to increase the success rate of external cephalic version.
Search strategy
This metaanalysis was performed according to a protocol recommended for systematic review. The review protocol was designed a priori defining methods for collecting, extracting and analyzing data. The research was conducted using MEDLINE, EMBASE, Web of Sciences, Scopus, ClinicalTrial.gov , OVID, and Cochrane Library as electronic databases. The trials were identified with the use of a combination of the following text words: external cephalic version, anesthesia, analgesia, spinal, epidural, anesthetic interventions, obstetric anesthesia, regional anesthesia, and randomized from the inception of each database to January 2016. No restrictions for language or geographic location were applied.
Study selection
We included all randomized clinical trials of women with breech and/or transverse presentation undergoing external cephalic version who were randomized to neuraxial analgesia, including spinal analgesia, epidural analgesia, or combined spinal-epidural technique (ie, intervention group) or to intravenous analgesia or no anesthetic treatment (control group). We therefore included both studies comparing neuraxial analgesia vs intravenous analgesia and studies comparing neuraxial analgesia vs no anesthetic intervention. Only women with gestational age at or greater than 36 weeks were included. Quasirandomized trials (ie, trials in which allocation was done on the basis of a pseudorandom sequence, eg odd/even hospital number or date of birth, alternation) were excluded.
Data extraction and risk of bias assessment
The risk of bias in each included study was assessed by using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions . Seven domains related to risk of bias were assessed in each included trial because there is evidence that these issues are associated with biased estimates of treatment effect including the following: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. Review authors’ judgments were categorized as low risk, high risk, or unclear risk of bias.
All analyses were done using an intention-to-treat approach, evaluating women according to the treatment group to which they were randomly allocated in the original trials. The primary outcome was successful external cephalic version, defined as the percentage of fetuses that were externally rotated from breech or transverse presentation to a vertex presentation at external cephalic version.
Secondary outcomes were incidence of cesarean delivery, vaginal delivery, vaginal breech delivery, emergency cesarean delivery, fetal morbidity (transient bradycardia and nonreassuring fetal testing after external cephalic version), maternal discomfort, maternal pain score, and incidence of abruption placentae.
Data from each eligible study were extracted without modification of original data onto custom-made data collection forms. Two authors (E.R.M.-M. and G.S.) independently assessed inclusion criteria, risk of bias, and data extraction. Disagreements were resolved by consensus through a discussion with a third reviewer (V.B.). Data not presented in the original publications were requested from the principal investigators.
We planned to assess the primary outcome (ie, successful external cephalic version) in subgroup analyses according to the type of control (either intravenous analgesia or no anesthetic intervention) and also according to the type of neuraxial technique (spinal vs epidural). We also performed a sensitivity analysis according to the risk of bias of the included trials.
Data analysis
The data analysis was completed independently by 2 authors (E.R.M.-M. and G.S.) using Review Manager 5.3 (The Nordic Cochrane Center, Cochrane Collaboration, 2014; Copenhagen, Denmark). The completed analyses were then compared, and any difference was resolved with review of the entire data and independent analysis.
Between-study heterogeneity was explored using the I 2 statistic, which represents the percentage of between-study variation that is due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, whereas I 2 values of ≥50% indicate a substantial level of heterogeneity. A fixed-effects model was used if substantial statistical heterogeneity was not present. On the contrary, if there was evidence of significant heterogeneity between studies included, a random-effect model was used. Potential publication biases were assessed statistically by using Begg’s and Egger’s tests. A value of P < .05 was considered statistically significant.
Tests for funnel plot asymmetry were carried out only with an exploratory aim when the total number of publications included for each outcome was less than 10. In this case, the power of the tests is too low to distinguish chance from real asymmetry. The summary measures were reported as relative risk or as mean differences with 95% confidence interval.
The metaanalysis was reported following the Preferred Reporting Item for Systematic Reviews and Meta-Analyses statement. Before data extraction, the review was registered with the International Prospective Register of Systematic Reviews (registration number CRD42016036363).
Results
Study selection and study characteristics
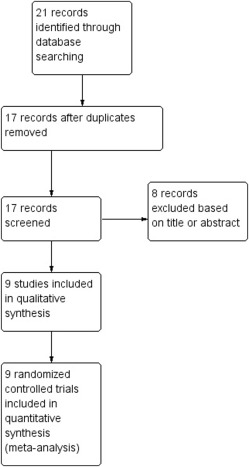
Figure 1 shows the flow diagram (Preferred Reporting Item for Systematic Reviews and Meta-Analyses template) of information derived from the reviewing of potentially relevant articles. Nine randomized clinical trials (934 women), meeting inclusion criteria, were included in this review. Two studies were published in abstract form only.
Tests for funnel plot asymmetry were carried out only with an exploratory aim because the total number of publications included for each outcome was less than 10. Despite this, the quality of the randomized clinical trials included in our metaanalysis assessed by the Cochrane Collaboration’s tool was high. All the included studies had low risk of bias in allocation concealment, random sequence generation, and incomplete outcome data. In 3 of the included randomized clinical trials, all investigators were blinded for anesthetic intervention to the randomization ( Figure 2 ).
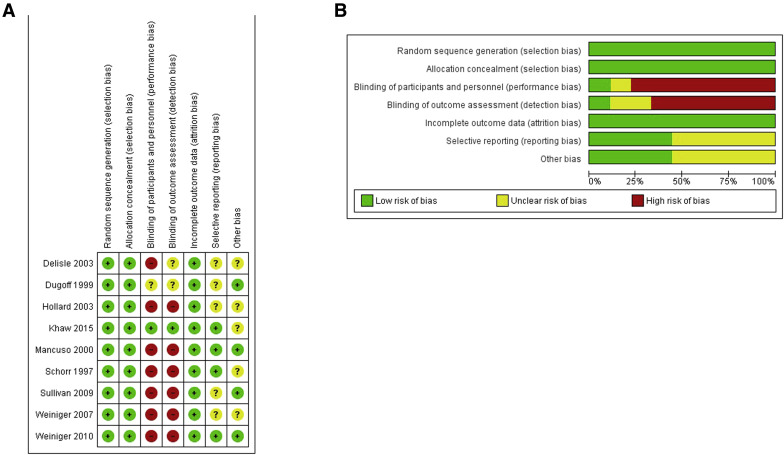
Publication bias, assessed using Begg’s and Egger’s tests, was not significant ( P = .77 and P = .64, respectively). Statistical heterogeneity within the trials was low, with no inconsistency in the risk estimate for the primary outcome. Unpublished data were provided by one author.
Table 1 shows the characteristics of the included trials. Table 2 shows inclusion and exclusion criteria. Characteristics of the women included (maternal age, gestational age at external cephalic version, parity and anterior placenta location) were reported in Table 3 . All studies randomized women with singleton breech or transverse presentations at term or late-preterm (≥36 weeks) and no contraindications to external cephalic version.
| Characteristics | Schorr et al, 1997 | Dugoff et al, 1999 | Mancuso et al, 2000 | Hollard et al, 2003 | Delisle et al, 2003 | Weiniger et al, 2007 | Sullivan et al, 2009 | Weiniger et al, 2010 | Khaw et al, 2015 |
|---|---|---|---|---|---|---|---|---|---|
| Study location | Mississippi | Colorado | Hawaii | California | British Columbia | Israel | Illinois | Israel | China |
| Sample size | 69 (35/34) | 102 (50/52) | 108 (54/54) | 36 (17/19) | 201 (99/102) | 70 (36/34) | 95 (48/47) | 64 (31/33) | 189 (63/63/63) a |
| Type of malpresentation | Breech 31/35 (88.6%) vs 29/34 (85.3%) transverse 4/35 (11.4%) vs 5/34 (14.7%) | Breech | Breech 50/54 (92.6%) vs 49/54 (90.7%) transverse 4/54 (7.4%) vs 5/54 (9.3%) | Breech | Nonvertex | Breech | Breech | Breech | Breech |
| GA at ECV, wks | >37 | >36 | ≥37 | >36 | >36 | 37–40 | ≥36 | 37–40 | At term |
| Regional analgesia (epidural and/or spinal) | Epidural, 2%, lidocaine with 1:200,000 epinephrine | Spinal, 10 μg sufentanil and 1 mL of 0.25% bupivacaine | Epidural, 2% lidocaine with 1:200,000 epinephrine and 100 μg of fentanyl | Spinal, 6 mg of 2% lidocaine and 15 μg fentanyl | Spinal, 2.5 mg bupivacaine and 20 μg fentanyl | Spinal, 7.5 mg bupivacaine | Spinal: 2.5 mg bupivacaine and 15 μg fentanyl Epidural: 45 mg lidocaine and 15 μg epinephrine | Spinal: 7.5 mg bupivacaine | Spinal: 9 mg bupivacaine and 15 μg remifentanil (group 1) |
| Control group | No anesthetic intervention | No anesthetic intervention | No anesthetic intervention | No anesthetic intervention | No anesthetic intervention | No anesthetic intervention | IVA | No anesthetic intervention | IVA (group 2) No anesthetic intervention (group 3) |
| IVA | — | — | — | — | — | — | 50 μg fentanyl | — | 0.1 μg/kg remifentanil |
| Blinded | All investigators were blinded | All investigators were blinded | Not reported | Operators were not blinded | Not reported | The 2 obstetricians were not blinded | Obstetricians were not blinded | The two obstetricians were not blinded | Operators were blinded |
| Tocolysis | 0.25 mg terbutaline, SC | 0.25 mg terbutaline i.v. | 0.25 mg terbutaline SC | 0.25 mg terbutaline SC | Nitroglycerin i.v. | Ritodrine 50 mg i.v./ nifedipine 20 mg SL b | 0.25mg Terbutaline i.v. | Ritodrine 50mg i.v. / nifedipine 20 mg SL b | 10 μg hexoprenaline i.v. |
| Hydration before anesthesia | 2000 mL Ringer’s solution | 500 mL Ringer’s solution | 1500 mL Ringer’s solution | 1000 mL Ringer’s solution | At the discretion of the attending anesthesiologist | 1000 mL Ringer’s solution | 500 mL Ringer’s solution | 1000 mL Ringer’s solution | 500 mL Hartmann’s solution |
| Number of maximum attempts at ECV | 3 | 4 | 3 | 2.1 ± 1.4 vs 2.6 ± 1.4 c | 4 | 3 | Not reported | 3 | 5 |
| Number of operators | Not reported | Not reported | 2 | 1 | Not reported | 2 | Not reported | 2 | 2 |
| Experience of the operator | Resident physician (third or fourth year) with a MFM fellow in attendance | Staff physician under direct supervision of attending physicians | Resident physician with assistance from experienced attending obstetricians | One MFM physician | Had at least a successful ECV in primiparous without regional anesthesia in the past or resident under supervision | Senior obstetrician (5 y experience with ECV) assisted by another obstetrician | Obstetricians | Senior obstetrician (5 y experience with ECV) assisted by another obstetrician | A pool of 5 obstetric specialists experienced in performing ECV |
| Primary outcome | Successful ECV | Successful ECV | Successful ECV | Successful ECV | Successful ECV | Successful ECV | Successful ECV | Successful ECV | Successful ECV |
| Level of anesthesia | Sensory at T6 | Sensory at T6 | Sensory at T10 | Not reported | Sensory at T6 | Sensory at T6 | Not reported | Sensory at T6 | Sensory at T7 |
| Other comments | Transvaginal elevation of the breech | Group 3 with failed ECV was further randomized to receive RA or IVA |
a Group 1/group2/group 3: group 1 received spinal analgesia, group 2 received IVA, and group 3 received no anesthetic intervention
b Ritodrine has been replaced with nifedipine because of nonavailability of this drug during the study
| Study | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Schorr et al, 1997 | GA >37 wks | Placenta previa, evidence of fetal compromise, IUGR, PROM |
| Dugoff et al, 1999 | GA ≥36 wks, breech presentation (not transverse or oblique lie); reactive NST; intact membranes with a minimum 2 × 2 cm pocket of amniotic fluid | Gross fetal anomalies, uterine malformation, EFW >4000 g, IUGR, placenta previa; known maternal history of third-trimester vaginal bleeding; labor, no contraindications to spinal anesthesia or terbutaline |
| Mancuso et al, 2000 | At least 18 y with singleton pregnancies of at least 37 wks in breech or transverse presentations, intact membranes, EFW between 2000 and 4000 g, reassuring FHR testing | Placenta previa, prior classical CD, third-trimester bleeding, AFI <5 cm or >25 cm, known uterine malformation, uncontrolled hypertension, suspected major fetal anomaly, active-phase labor |
| Hollard et al, 2003 | Singleton gestation, breech presentation, GA >36 wks, not in labor, reactive fetal heart rate | Uteroplacental insufficiency, third-trimester bleeding, IUGR, AFI <6, uterine malformations, placenta previa, maternal cardiac or hypertensive disease, PROM, fetal anomaly, EFW >4500 g, previous uterine surgery, maternal obesity >50% of IBW |
| Delisle et al, 2003 | Singleton fetuses in a nonvertex presentation, maternal age of at least 18, GA ≥36 wks, intact membranes, reactive NST | Not reported in abstract |
| Weiniger et al, 2007 | ASA status I-II, GA 37–40 wks, no fetal abnormality | Previous uterine surgery or uterine anomaly, contraindication for vaginal delivery and for regional analgesia, patient refusal of regional analgesia, neuropathy, severe back pain with neurological radiation, poor communication, BMI ≥40 kg/m 2 |
| Sullivan et al, 2009 | GA ≥36 wks, singleton pregnancies, willing to receive either CSE analgesia or systemic opioid analgesia for ECV | Contraindications to neuraxial anesthesia or allergies to any study medication |
| Weiniger et al, 2010 | ASA status I-II, GA 37–40 wks, no fetal abnormality (including IUGR), no contraindication for vaginal delivery and for regional analgesia | Previous CD, previous myomectomy with uterine cavity penetration or uterine anomaly, BMI ≥40 kg/m 2 , AFI <7 cm, neuropathy, severe back pain with radicular radiation, request for elective CD |
| Khaw et al, 2015 | ASA status I-II, term parturients, breech-presenting fetus | Contraindications to ECV including patients with known uterine scar or anomaly, unexplained third-trimester bleeding, obstetric or medical conditions complicating pregnancy, compromised fetus, nuchal cord, fetal anomaly, PROM, labor |
| Characteristics | Schorr et al, 1997 | Dugoff et al, 1999 | Mancuso et al, 2000 | Hollard et al, 2003 | Delisle et al, 2003 | Weiniger et al, 2007 | Sullivan et al, 2009 | Weiniger et al, 2010 | Khaw et al, 2015 |
|---|---|---|---|---|---|---|---|---|---|
| Maternal age, y, mean ± SD or median [range] | 27.7 ± 6.1 vs 25.8 ± 6.6 | 24.3 ± 0.9 vs 26.8 ± 0.9 | 28.5 ± 4.8 vs 28.2 ± 4.8 | 29.8 ± 4.9 vs 29.3 ± 4.9 | Not reported a | 24.6 ± 3.8 vs 28.1 ± 4.1 | 32 [27–35] vs 33 [30–36] | 28.5 [21–40] vs 28.6 [20–36] | 32 [23–42] vs 32 [20–42] |
| GA at ECV, wks, mean ± SD or median [range] | 38.0 ± 2.3 vs 37.4 ± 2.1 | 38.0 ± 0.2 vs 38.0 ± 0.2 | 38.1 ± 1.2 vs 37.9 ± 1.0 | 38.0 ± 8 vs 37.4 ± 5 | 37.4 (37.2–37.4) vs 37.2 (37.0–37.5) b | 37.9 ± 1.0 vs 37.9 ± 1.0 | 37 [37–38] vs 37 [37–38] | 38.1 ± 0.9 vs 38.2 ± 1.1 | 36.9 [36.6–39.2] vs 36.6 [36.1–39.6] |
| Multiparous n, %, or mean ± SD or median [range] | 21/35 (60%) vs 18/34 (53%) | 1.5 ± 0.0 vs 1.6 ± 0.1 | 24/54 (44%) vs 25/54 (46%) | 9/17 (53%) vs 13/19 (68%) | 39/99 (39.4%) vs 39/102 (38.2%) | 0/36 vs 0/34 | 18/48 (62%) vs 18/47 (38%) | 31/31 (100%) vs 33/33 (100%) | 1 [0–3] vs 1 [0–4] |
| Anterior placenta, n, % | 13/35 (37%) vs 11/34 (32%) | 23/50 (46%) vs 22/52 (42%) | 20/54 (37%) vs 18/54 (33%) | 6/17 (35%) vs 10/19 (53%) | Not reported | 14/36 (39%) vs 14/34 (41%) | Not reported | 11/31 (35%) vs 17/33 (51%) | Not reported |
a Delisle et al reported a similar maternal age in both groups
All randomized clinical trials used a tocolytic drug in both groups and hydration before the anesthetic intervention. Tocolytic therapy differed in the type of agent used and in route of administration: 3 trials used subcutaneous terbutaline, 2 trials used intravenous terbutaline, 1 used intravenous hexoprenaline, 1 used intravenous nitroglycerine, and 2 studies used intravenous ritodrine, which was replaced with sublingual nifedipine after 8 months because of nonavailability of this drug during the study.
One study compared neuraxial analgesia with intravenous analgesia ; 7 trials compared neuraxial analgesia with no anesthetic intervention. The trial of Khaw et al was a double-phased, 3-armed randomized blinded study: in phase I, 189 women were randomized to external cephalic version under either spinal anesthesia, intravenous analgesia, or no anesthetic interventions (control group); in phase II, women in the control group in whom the initial external cephalic version failed were further randomized to receive either spinal analgesia (n = 9) or intravenous analgesia (n = 9) for a reattempt. We therefore excluded the phase II from our metaanalysis.
Regarding the intervention, 6 studies addressed the effect of spinal analgesia on external cephalic version ; 2 studies assessed the effect of epidural. Sullivan et al used a combined spinal-epidural technique.
Of the 934 singletons included in the metaanalysis, 433 (46.4%) were randomized to the neuraxial analgesia group (ie, intervention group) and 501 (53.6%) to the control group (either intravenous analgesia or no anesthetic intervention).
Synthesis of results
Table 4 shows the pooled data of the primary and the secondary outcomes of the metaanalysis. Women who received neuraxial techniques had a significantly higher incidence of successful external cephalic version (58.4% vs 43.1%; relative risk, 1.44, 95% confidence interval, 1.27–1.64) ( Figure 3 ), cephalic presentation in labor (55.1% vs 40.2%; relative risk, 1.37, 95% confidence interval, 1.08–1.73), and vaginal delivery (54.0% vs 44.6%; relative risk, 1.21, 95% confidence interval, 1.04–1.41) compared with those who did not.
| Outcomes | Schorr et al, 1997 | Dugoff et al, 1999 | Mancuso et al, 2000 | Hollard et al, 2003 | Delisle et al, 2003 | Weiniger et al, 2007 | Sullivan et al, 2009 | Weiniger et al, 2010 | Khaw et al, 2015 | Total | I 2 | RR or MD (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Successful ECV | 24/35 (68.6%) vs 11/34 (32.3%) | 22/50 (44.0%) vs 22/52 (42.3%) | 32/54 (59.2%) vs 18/54 (33.3%) | 9/17 (52.9%) vs 10/19 (52.6%) | 41/99 (41.4%) vs 31/102 (33.7%) | 24/36 (66.6%) vs 11/34 (32.3%) | 22/48 (45.8%) vs 14/47 (30.0%) | 27/31 (87.0%) vs 19/33 (57.6%) | 52/63 (82.5%) vs 80/126 (63.5%) | 253/433 (58.4%) vs 216/501 (43.1%) | 16% | 1.44 (1.27–1.64) |
| Cephalic presentation in labor | 24/35 (68.6%) vs 10/34 (29.4%) | 20/50 (40%) vs 26/52 (50%) | 32/54 (59.2%) vs 19/54 (35.2%) | 10/17 (58.8%) vs 9/19 (47.4%) | Not reported | Not reported | Not reported | Not reported | Not reported | 86/156 (55.1%) vs 64/159 (40.2%) | 75% | 1.37 (1.08–1.73) |
| Vaginal delivery | 23/35 (65.7%) vs 7/34 (20.6%) | 16/50 (32.0%) vs 25/52 (48.1%) | 29/54 (53.7%) vs 17/54 (31.5%) | 9/17 (52.9%) vs 8/19 (42.1%) | Not reported | Not reported | 17/48 (36.0%) vs 12/47 (25.0%) | 27/31 (87.1%) vs 30/33 (91.0%) | 40/63 (63.5%) vs 64/126 (51.0%) | 161/298 (54.0%) vs 163/365 (44.6%) | 91% | 1.21 (1.04–1.41) |
| Vaginal breech delivery | Not reported | 0/50 (0.0%) vs 0/52 (0.0%) | 1/54 (1.8%) vs 3/54 (5.5%) | 0/17 (0.0%) vs 0/19 (0.0%) | Not reported | Not reported | 0/48 (0.0%) vs 0/47 (0.0%) | 0/31 (0.0%) vs 3/33 (9.1%) | Not reported | 1/102 (0.98%) vs 6/106 (5.6%) | 0% | 1.17 (0.02–1.41) |
| CD | 12/35 (34.3%) vs 27/34 (79.4%) | 34/50 (68.0%) vs 27/52 (51.9%) | 25/54 (46.3%) vs 37/54 (68.5%) | 8/17 (47.0%) vs 11/19 (57.9%) | Not reported | Not reported | 31/48 (64.0%) vs 35/47 (75.0%) | 4/31 (12.9%) vs 3/33 (9.1%) | 23/63 (36.5%) vs 62/126 (49.2%) | 137/298 (46.0%) vs 202/365 (55.3%) | 75% | 0.83 (0.71–0.97) |
| Emergency CD within 24 hours of ECV | Not reported | 0/50 (0.0%) vs 1/52 (1.9%) | 0/54 (0.0%) vs 0/54 (0.0%) | 1/17 (5.9%) vs 0/19 (0.0%) | 1/73 (1.4%) vs 0/68 (0.0%) | 0/36 (0.0%) vs 0/34 (0.0%) | 1/48 (2.1%) vs 1/47 (2.1%) | 0/31 (0.0%) vs 0/33 (0.0%) | 3/63 (4.8%) vs 9/126 (7.14%) | 6/372 (1.6%) vs 11/433 (2.5%) | 0% | 0.63 (0.24–1.70) |
| Transient bradycardia | Not reported | 11/50 (22.0%) vs 6/52 (12.0%) | 2/54 (3.7%) vs 3/54 (5.5%) | 3/17 (17.6%) vs 3/19 (15.8%) | Not reported | Not reported | Not reported | 2/31 (6.4%) vs 1/33 (3.0%) | Not reported | 18/152 (11.8%) vs 13/156 (8.3%) | 45% | 1.42 (0.72–2.80) |
| Nonreassuring fetal testing (excluding transient bradycardia) after ECV | Not reported | 0/50 (0.0%) vs 1/52 (1.9%) | Not reported | 1/17 (5.9%) vs 0/19 (0.0%) | 1/73 (1.4%) vs 0/68 (0.0%) | 2/36 (5.5%) vs 0/34 (0.0%) | 14/48 (29.2%) vs 13/47 (27.6%) | 1/31 (3.0%) vs 0/33 (0.0%) | 3/63 (4.8%) vs 9/126 (7.1%) | 22/318 (6.9%) vs 23/311 (7.4%) | 15% | 0.93 (0.53–1.64) |
| Maternal discomfort | 1/35 (2.8%) vs 4/34 (11.8%) | 0/50 (0.0%) vs 4/52 (8.0%) | Not reported | Lower in RA group | Not reported | Lower in RA group | Lower in RA group | Lower in RA group | Lower in RA group | 1/85 (1.2%) vs 8/86 (9.3%) | 0% | 0.12 (0.02–0.99) |
| Maternal pain score | Not reported | Not reported | Not reported | 2.3 ± 2.6 vs 7.2 ± 2.8 a | Not reported | 1.76 ± 2.7 vs 6.84 ± 3.1 a | 3 [0–12] vs 36 [16–54] b | 1.7 ± 2.4 vs 5.5 ± 2.9 a | 0 [0–0] vs 35 [0–60] vs 50 [30–75] b | — | 0% | –4.52 point (–5.35 to 3.69) |
| Abruption placentae | 0/35 (0.0%) vs 0/34 (0.0%) | 0/50 (0.0%) vs 1/52 (1.9%) | 0/54 (0.0%) vs 0/54 (0.0%) | 1/17 (5.9%) vs 0/19 (0.0%) | Not reported | 0/36 (0.0%) vs 0/34 (0.0%) | Not reported | 0/31 (0.0%) vs 0/33 (0.0%) | Not reported | 1/223 (0.4%) vs 1/226 (0.4%) | 0% | 1.01 (0.06–16.1) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


