Chapter 93 Nervous System Disorders
93.1 The Cranium
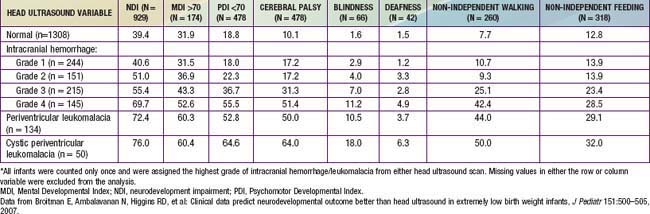
Erythema, abrasions, ecchymoses, and subcutaneous fat necrosis of facial or scalp soft tissues may be noted after a normal delivery or after forceps or vacuum-assisted deliveries. Their location depends on the area of contact with the pelvic bones or of application of the forceps. Traumatic hemorrhage may involve any layer of the scalp as well as intracranial contents (Fig. 93-1).
Caput succedaneum is a diffuse, sometimes ecchymotic, edematous swelling of the soft tissues of the scalp involving the area presenting during vertex delivery (see Fig. 93-1). It may extend across the midline and across suture lines. The edema disappears within the 1st few days of life. Molding of the head and overriding of the parietal bones are frequently associated with caput succedaneum and become more evident after the caput has receded; they disappear during the 1st weeks of life. Rarely, a hemorrhagic caput may result in shock and require blood transfusion. Analogous swelling, discoloration, and distortion of the face are seen in face presentations. No specific treatment is needed, but if extensive ecchymoses are present, hyperbilirubinemia may develop.
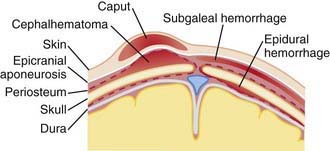
Cephalohematoma (Fig. 93-2) is a subperiosteal hemorrhage, hence always limited to the surface of one cranial bone. Cephalohematomas occur in 1-2% of live births. No discoloration of the overlying scalp occurs, and swelling is not usually visible for several hours after birth because subperiosteal bleeding is a slow process. The lesion becomes a firm tense mass with a palpable rim localized over one area of the skull. Most cephalohematomas are resorbed within 2 wk-3 mo, depending on their size. They may begin to calcify by the end of the 2nd week. A few remain for years as bony protuberances and are detectable on radiographs as widening of the diploic space; cystlike defects may persist for months or years. An underlying skull fracture, usually linear and not depressed, may be associated with 10-25% of cases. A sensation of central depression suggesting but not indicative of an underlying fracture or bony defect is usually encountered on palpation of the organized rim of a cephalohematoma. Cephalohematomas require no treatment, although phototherapy may be necessary to treat hyperbilirubinemia. Infection of the hematoma is a very rare complication.
93.3 Intracranial-Intraventricular Hemorrhage and Periventricular Leukomalacia
Clinical Manifestations
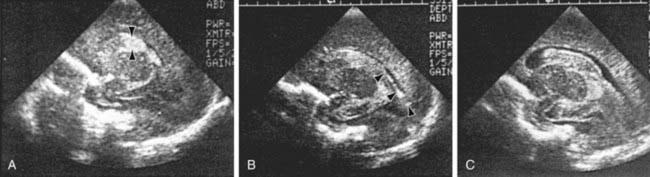
The severity of hemorrhage may be defined on CT scans by the location and degree of ventricular dilatation. In a grade I hemorrhage, bleeding is isolated to the subependymal area. In Grade II hemorrhage, there is bleeding within the ventricle but without evidence of ventricular dilatation. Grade III hemorrhage consists of IVH with ventricular dilatation. In Grade IV hemorrhage, there is intraventricular and parenchymal hemorrhage. Another grading system describes 3 levels of increasing severity of IVH detected on ultrasound: In grade I, bleeding is confined to the germinal matrix–subependymal region or to <10% of the ventricle (≈35% of IVH cases); grade II is defined as intraventricular bleeding with 10-50% filling of the ventricle (≈40% of IVH cases) and in grade III, more than 50% of the ventricle is involved, with dilated ventricles (Fig. 93-3). Ventriculomegaly is defined as mild (0.5-1 cm), moderate (1.0-1.5 cm), or severe (>1.5 cm).
Prognosis
The degree of IVH and the presence of PVL are strongly linked to neurodevelopmental impairment. For infants with birthweight <1,000 g, the incidences of severe neurologic impairment (defined as mental developmental index <70, psychomotor development index <70, cerebral palsy, blindness, or deafness) are about 50%, 55%, and 70% for infants with grade II, grade III, and grade IV IVH, respectively (Table 93-1). In contrast, the rate of neurodevelopmental impairment is approximately 40% in infants without IVH and those with grade I IVH. PVL, cystic PVL, and progressive hydrocephalus requiring shunt insertion are each independently associated with a poorer prognosis.
Table 93-1 PERCENTAGE OF INFANTS WITH EACH NEUROLOGIC OUTCOME AT 18 TO 22 MONTHS CORRECTED AGE BY HEAD ULTRASOUND FINDINGS*