CHAPTER 9 Nerve repair/reconstruction strategies for neonatal brachial plexus palsies
Summary Box
Selecting patients for surgery
The appropriate selection of patients with neonatal brachial plexus palsy (NBPP) who may benefit from surgical intervention remains controversial, and several different paradigms have been reported.1–3 As a prerequisite for understanding this chapter’s discussion regarding nerve repair and reconstruction strategies, we describe the current surgical selection process at the Leiden University Medical Center. We seek to identify all patients with neurotmetic lesions or nerve root avulsions as surgical candidates. We consider absent or significantly impaired hand function, in the context of a flail arm at birth, to be an absolute indication for nerve surgery as soon as the infant reaches the age of 3 months.4 Similarly, we recommend operative intervention to NBPP patients who demonstrate no spontaneous recovery of shoulder external rotation and elbow flexion/forearm supination by 3–4 months of age. If the presence of true shoulder and elbow movements is doubtful, we proceed with surgical exploration, because the potential benefits from repairing neurotmetic lesions generally outweigh the risks of negative exploration. Surgery for NBPP is rarely performed before 3 months of age and is almost always performed before 7 months of age.
In our patient selection process, we try to assess severity of the brachial plexus lesion(s) as early as possible for surgical and psychosocial reasons, and to give parents/caretakers the time needed to consider the recommended treatment options. We proposed a paradigm for identifying severe nerve lesions at 1 month of age as a result of our prospective study.5 Elbow extension and elbow flexion are clinically assessed and needle electromyography (EMG) of the biceps muscle is performed. Severe lesions of C5 and C6/upper trunk can be predicted in the vast majority of infants at 1 month of age in whom elbow extension is absent or in whom both elbow flexion and motor unit action potentials (MUAP) are absent in the biceps muscle. Furthermore, radiographic assessment via ultrasound of diaphragm (to detect phrenic nerve palsy) and CT-myelography (to detect nerve root avulsions) can provide additional evidence for severe NBPP lesions that are amenable to surgical repair/reconstruction.6,7
Surgical procedure
Supraclavicular exposure
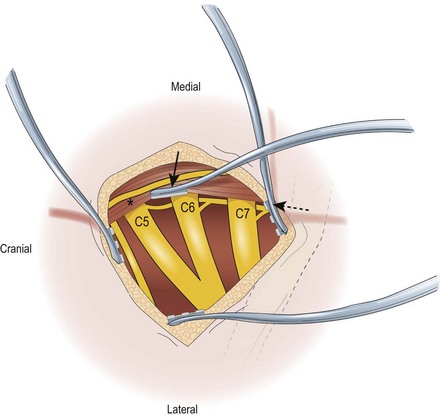
The lateral margin of the sternocleidomastoid muscle is identified, with its sternal and clavicular heads. The lateral aspect of the clavicular head is released to facilitate exposure. The supraclavicular nerves (sensory nerves branches of the ansa cervicalis, C2–C4) are identified along their superficial cranial-caudal course. These nerves are likewise preserved for anatomical landmarks and for intraplexal transfers and, occasionally, for potential donors for nerve graft material. The supraclavicular nerves are followed proximally until the C4 spinal nerve root is identified. The cervical fascia/scalene fat pad is released from this parallel to the sternocleidomastoid muscle starting at the level of C4 in a cranial to caudal direction; at the retroclavicular level, a 90-degree turn parallel to the clavicle. The resulting cervical fascia/scalene fat pad can be mobilized for the operation then replaced at closure to cover the nerve grafts and coaptation sites after the reconstruction. The cervical fascia/scalene fat pad should be preserved as much as possible; i.e., coagulation should be avoided, as the vascular fat pad may contribute to revascularization of nerve grafts and may provide the optimal environment for the nerve elements. When releasing the fat pad during exposure of the left supraclavicular brachial plexus, one should preserve or ligate the thoracic duct to avoid chyle leakage. The transverse cervical artery and vein that run parallel to the clavicle ventral to the brachial plexus elements are retracted or ligated. The omohyoid muscle is identified between the superficial and deep cervical fascia along its course toward the suprascapular notch, and it can be tagged and retracted. Note that preserving this muscle to identify the suprascapular notch permits identification of the suprascapular nerve (SSN), especially in patients whose anatomy is distorted by trauma. Appropriate placement of intraoperative retractors can facilitate surgical exposure of the supraclavicular brachial plexus (Figure 9.1).
Exposure of extraplexal nerves for nerve transfer procedures
Another commonly used donor nerve is the medial pectoral nerve (MPN); it is used for nerve transfer to the MCN for restoration of elbow flexion. The MCN can be identified in its course dorsal to the pectoralis major and minor muscles. Generally MPNs can be reached by retracting the pectoralis major muscle cranially through a low incision in the deltopectoral groove. The MPN originates from the medial cord, and its function remains intact in C5–C6 or C5/C6/C7 lesions.8 Intraoperative nerve stimulation is an indispensable step for identification of MPNs, because small vessels simulate their appearance and course. There are usually 2 individual MPN branches, and they should be cut as distally as possible then coapted to the MCN. The total cross-sectional area of the MPN branches may be less than that of the MCN, and if so, coaptation to a fascicle of the MCN is undertaken.
Another nerve transfer technique for restoration of elbow flexion uses intercostal nerves (ICNs) as donors and the MCN as recipient. We previously described the technique for ICN transfer in adults.9 We apply the same surgical technique in infants with NBPP. ICN 3-6 are exposed by means of an undulating skin incision over the ipsilateral chest. The incision starts at the anterior axillary line at the inferior border of the pectoralis major muscle and continues beneath the nipple, extending medially to the costosternal junction. The inferior part of the pectoralis major muscle is shifted upward, with partial detachment of its sternal insertion, if necessary. The rib attachments of the serratus anterior muscle usually remain intact. The main branch of the ICN is identified halfway in its ventral course between the external and internal intercostal muscles and dissected free over its entire anterior course. Care should be taken to keep the periosteum of the ribs intact in order to avoid rib cage deformities during growth. ICN motor responses are assessed using electrical nerve stimulation. If feasible, sensory branches are identified by their course toward the skin and left intact after they have been interfascicularly dissected from the main nerve. The ICNs are then transected as close as possible to the sternum to obtain sufficient length for direct coaptation to the MCN and are tunneled to the axilla. The infraclavicular and intercostal wounds remain separated from each other by an area of intact skin at the anterior axilla, facilitating wound closure and healing. In female infants, if the anatomical localization of sensory innervation to the nipple is uncertain, the third ICN is left untouched to preserve at least partial sensation to the breast. The MCN is cut proximally after freeing it from the lateral cord until fascicular intermingling is encountered. No attempt is made to identify the motor branches within the MCN. Before coaptation, the infant’s arm is abducted 90 degrees. The ICNs are coapted to the centrally located MCN fascicles by means of fibrin glue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree