CHAPTER 15 Nerve repair/nerve transfer strategies for adult brachial plexus palsies
Summary box
Introduction
Incidence
Brachial plexus injuries comprise approximately one-third of peripheral nerve injuries and are seen in slightly more than 1% of patients presenting to a trauma facility.1 Most of these injuries (>60%) are located at the supraclavicular plexus and are more likely (52%) to require surgery due to the severity of these injuries. Infraclavicular lesions are less likely (17%) to be operated on, with half of these cases found to have neurapraxic injuries. Of the supraclavicular plexal injuries having surgery due to the lack of clinical recovery, 67% will have involvement of lower plexal (C8 and T1 spine nerve) elements. Half of these with pan-plexal involvement will have avulsed some spinal nerves, and exceedingly few will regain any useful function without intervention. On the contrary, in patients whose lower plexal elements are spared and the primary injury is less extensive, up to 25% will have good functional recovery of involved elements after neurolysis and without nerve repair.2
Classification
The basic principles for management of closed traumatic brachial plexus palsies can also be applied to non-traumatic cases. Most brachial plexus palsies are trauma-related, with stretch-contusion injuries having the worst prognosis.3 Penetrating and lacerating injuries are managed a bit differently, as are tumor and compression or ischemic-type lesions. Inflammatory lesions that may involve plexus elements, such as Parsonage-Turner syndrome, are rarely managed surgically.
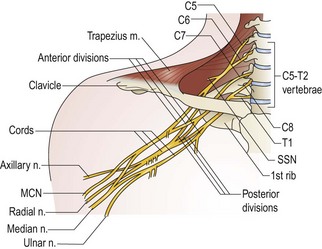
Injury patterns can be separated artificially into supraclavicular and infraclavicular injuries. The supraclavicular plexus refers to the C5–T1 spinal nerves and the upper, middle, and lower trunks with their branches and divisions (Figure 15.1). Common injury patterns involving upper plexus elements are “Erb“ (predominantly C5+C6) and “Erb plus” (C5–7) palsies. Lower plexus elements (C8+T1) are rarely involved in isolation and are more likely to be avulsed.
Goals and priorities
Generally, the main achievable objectives in treating severe brachial plexus injuries are to restore shoulder abduction, external rotation, elbow flexion, and forearm supination.4 The combination of these movements allows the patient to hold a food tray, bring his hand to his mouth, and to push doors open while carrying items with the healthy arm. Some experts also advocate attempting restoration of wrist extension and shoulder adduction. Restoration of sensation is a secondary objective and primarily directed to the median nerve distribution. Unfortunately, intrinsic hand function is more difficult to restore, with generally poor outcomes when repairing or grafting to lower trunk elements. Therefore, in view of the limited available options, focus should be directed toward the more reliably attainable goals listed above. It is essential that the surgical strategy be planned thoughtfully to maximize functional outcome. It is also vital that joint range of motion be preserved for the return of function. Within the limited time-window that exists to facilitate reinnervation of key muscle groups, there is usually no second opportunity to attain this if the first attempt should fail.
Surgical strategies
Strategies for surgical treatment can be grouped into 5 broad categories:
Suture (end-to-end) repair
This is the ideal in cases of focal injury, when nerve ends can be re-approximated without tension.5 It is the goal at early exploration of sharp penetrating injuries (within 72 hours). Few surgeons advocate implantation of avulsed spinal nerve rootlets.
Graft (direct) repair
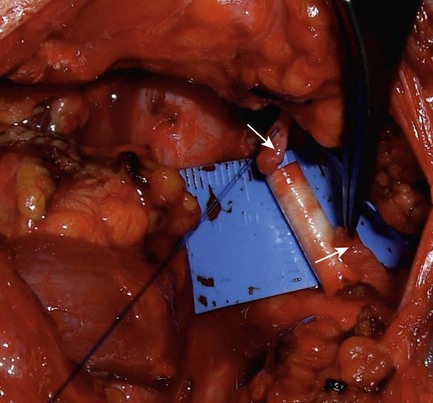
A gap must be breached between 2 unscarred nerve ends to provide a conduit for regenerating axons when regenerating NAPs are negative. Sural nerve or local expendable sensory nerves are used for long gaps. For short gaps up to 3 cm, commercially available tubular conduits may be used (Figure 15.2).6,7
Salvage procedures
Tendon and free muscle transfers, occasionally with shoulder arthrodesis, can be performed with reasonable success.8,9 Unlike nerve repair and transfer techniques, timing of these procedures is less important and is usually delayed until no further recovery is expected. Limb amputation is now rarely required. There is also an emerging field of bionics that may offer additional options in the future.
Currently there are no absolute guidelines when direct plexo-plexal repairs are not possible or are contraindicated, although Table 15.1 reflects some practical considerations. We consider the following to be appropriate conditions for performing nerve transfers:
Table 15.1 Relative contraindications for exploration and direct repair of plexus stretch injuries
The following are some useful criteria when choosing donor nerves for transfer: (1) an expendable or redundant donor nerve; (2) donor nerve is as close to the target end-organ as possible; (3) donor nerve with large number of predominantly motor axons for motor transfer, or sensory axons for sensory transfer; (4) donor nerve with synergistic action to the target muscle (when possible) to facilitate motor re-education;10 (5) size matching between donor and recipient nerves.
Preferred techniques and alternatives
Supraclavicular injuries
C5, C6, (C7) lesions [Erb’s, Erb’s plus]
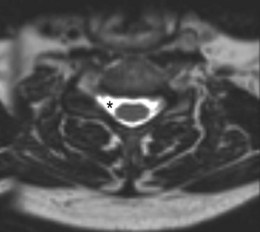
MRI of his cervical spine revealed a pseudomeningocele at the C4/5 level on the right side with asymmetry of nerve rootlets, suggesting a proximal injury to the C5 spinal nerve (Figure 15.3).
Intraoperative reasoning. We found no indication of C5 or C6 regeneration, and intraoperative findings were consistent with very proximal injury. NAPs were positive and abnormally large and fast, consistent with a pure preganglionic injury (avulsion) (Table 15.2). This should not be confused with regeneration across the zone of injury, when evoked potentials may also be recordable.
Table 15.2 Repair preferences for C5/C6 ± C7 stretch injuries
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|