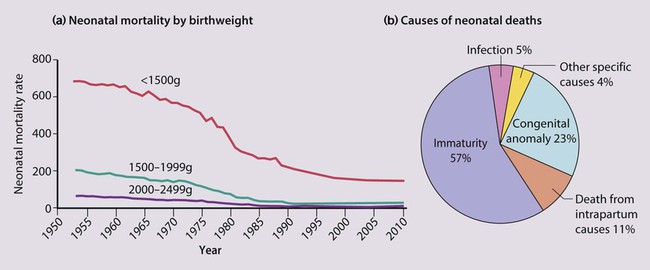
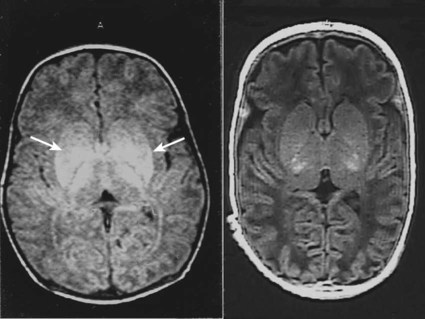
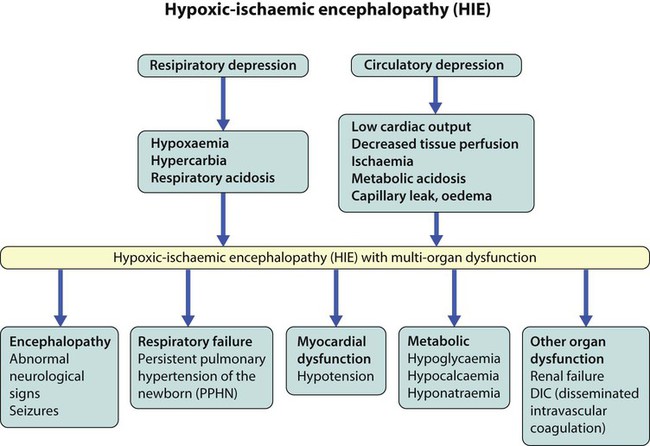
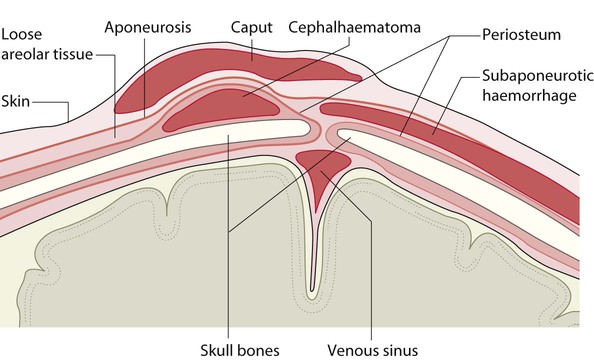
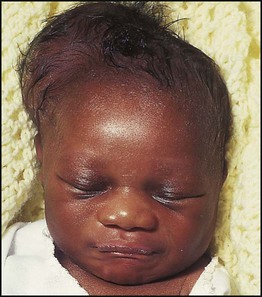
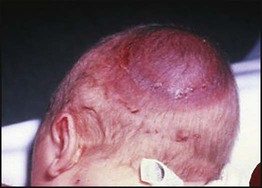
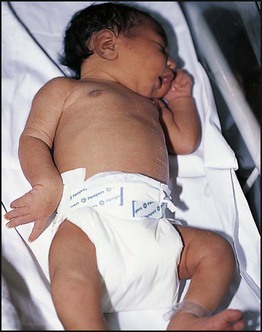
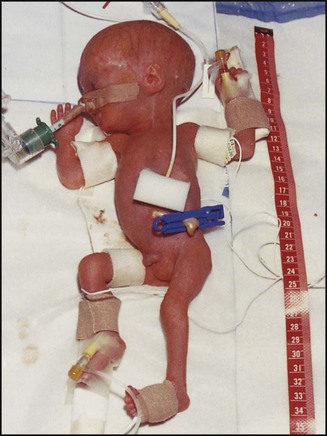
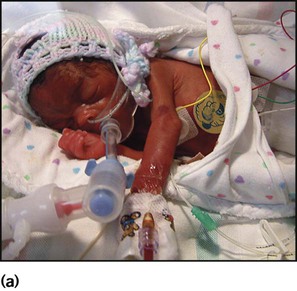
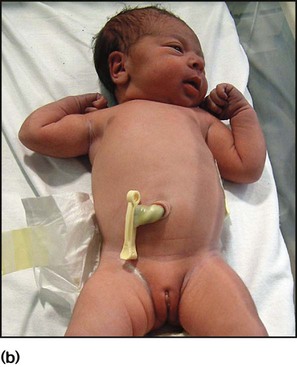
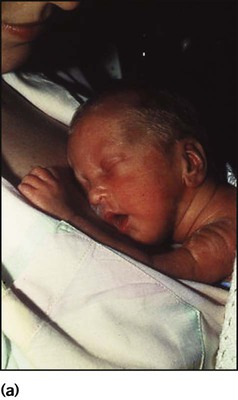
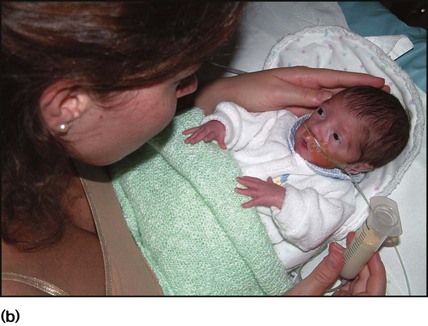
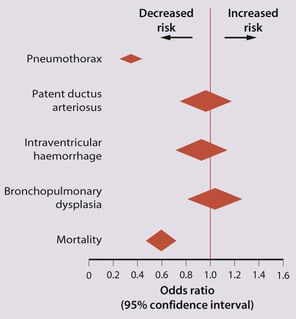
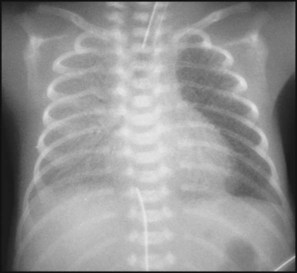
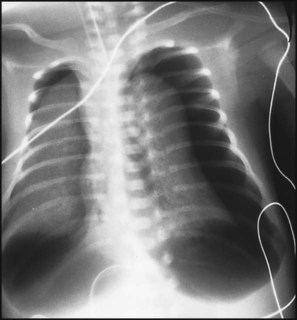
The dramatic reduction in neonatal mortality throughout the developed world has resulted from advances in the management of newborn infants together with improvements in maternal health and obstetric care. Neonatal intensive care became increasingly available in the UK from 1975, and it is since that time that the mortality of very low birthweight (VLBW) infants has fallen (Fig. 10.1). In the UK, neonatal units are organised as networks, with units providing either: • short-term intensive care (level 2) • long-term intensive care (level 3), usually linked to specialist fetal and obstetric care to form a specialist tertiary perinatal centre. In perinatal asphyxia, gas exchange, either placental or pulmonary, is compromised or ceases altogether, resulting in cardiorespiratory depression. Hypoxia, hypercarbia and metabolic acidosis follow. Compromised cardiac output diminishes tissue perfusion, causing hypoxic-ischaemic injury to the brain and other organs. The neonatal condition is called hypoxic-ischaemic encephalopathy (HIE). It remains an important cause of brain damage, resulting in disability (Fig. 10.2) or death, and its prevention is one of the key aims of modern obstetric care. In developed countries, approximately 0.5–1/1000 liveborn term infants develop HIE and 0.3/1000 have significant neurologic disability. The incidence is higher in developing countries. • Failure of gas exchange across the placenta – excessive or prolonged uterine contractions, placental abruption, ruptured uterus • Interruption of umbilical blood flow – cord compression including shoulder dystocia, cord prolapse • Inadequate maternal placental perfusion, maternal hypotension or hypertension – often with intrauterine growth restriction • Compromised fetus – anemia, intrauterine growth restriction • Failure of cardiorespiratory adaptation at birth – failure to breathe. The clinical manifestations start immediately or up to 48 h after asphyxia, and can be graded: • Mild – the infant is irritable, responds excessively to stimulation, may have staring of the eyes and hyperventilation and has impaired feeding • Moderate – the infant shows marked abnormalities of tone and movement, cannot feed and may have seizures • Severe – there are no normal spontaneous movements or response to pain; tone in the limbs may fluctuate between hypotonia and hypertonia; seizures are prolonged and often refractory to treatment; multi-organ failure is present. • recording of amplitude-integrated electroencephalogram (aEEG, cerebral function monitor) to detect abnormal background activity to confirm early encephalopathy or identify seizures • treatment of clinical seizures with anticonvulsants • fluid restriction because of transient renal impairment • treatment of hypotension by volume and inotrope support • monitoring and treatment of hypoglycaemia and electrolyte imbalance, especially hypocalcaemia. When HIE is mild, complete recovery can be expected. Infants with moderate HIE who have recovered fully on clinical neurological examination and are feeding normally by 2 weeks of age have an excellent long-term prognosis, but if clinical abnormalities persist beyond that time, full recovery is unlikely. Severe HIE has a mortality of 30–40%, and, of the survivors, over 80% have neurodevelopmental disabilities, particularly cerebral palsy. If magnetic resonance imaging (MRI) at 4–14 days in a term infant shows significant abnormalities (bilateral abnormalities in the basal ganglia and thalamus and lack of myelin in the posterior limb of the internal capsule), there is a very high risk of later cerebral palsy (Fig. 10.3). • evidence of severe hypoxia antenatally or during labour or at delivery • resuscitation needed at birth • evidence of hypoxic damage to other organs such as liver, kidney, or heart • no other prenatal or postnatal cause identified • Caput succedaneum (Fig. 10.4) – bruising and oedema of the presenting part extending beyond the margins of the skull bones; resolves in a few days • Cephalhaematoma (Figs 10.4, 10.5) – haematoma from bleeding below the periosteum, confined within the margins of the skull sutures. It usually involves the parietal bone. The centre of the haematoma feels soft. It resolves over several weeks • Chignon (Fig. 10.6) – oedema and bruising from Ventouse delivery • Bruising to the face after a face presentation and to the genitalia and buttocks after breech delivery. Preterm infants bruise readily from even mild trauma • Abrasions to the skin from scalp electrodes applied during labour or from accidental scalpel incision at Caesarean section • Forceps marks to face from pressure of blades – transient • Subaponeurotic haemorrhage (Fig. 10.4) (very uncommon) – diffuse, boggy swelling of scalp on examination, blood loss may be severe and lead to hypovolaemic shock and coagulopathy. Brachial nerve palsy results from traction to the brachial plexus nerve roots. They may occur at breech deliveries or with shoulder dystocia. Upper nerve root (C5 and C6) injury results in an Erb palsy (Fig. 10.7). It may be accompanied by phrenic nerve palsy causing an elevated diaphragm. Most palsies resolve completely, but should be referred to an orthopaedic or plastic surgeon if not resolved by 2–3 months. Most recover by 2 years. A facial nerve palsy may result from compression of the facial nerve against the mother’s ischial spine. It is unilateral, and there is facial weakness on crying but the eye remains open. It is usually transient, but methylcellulose drops may be needed for the eye. Rarely, nerve palsies may be from damage to the cervical spine, when there is lack of movement below the level of the lesion. The appearance, the likely clinical course, chances of survival and long-term prognosis depend on the gestational age at birth. The appearance and maturational changes of very preterm infants are described in Table 10.1 and the importance of parental involvement shown in Figures 10.10a and b. The external appearance and neurological findings can be scored to provide an estimate of an infant’s gestational age (see Appendix). The rate and severity of problems associated with prematurity decline markedly with increasing gestation. Infants born at 23–26 weeks’ gestation encounter many problems (Box 10.1), require many weeks of intensive and special care in hospital and have a high overall mortality. With modern intensive care, the prognosis is excellent after 32 weeks’ gestational age. The severity of an infant’s respiratory disease and of any episodes of infection largely determine the neonatal course and outcome. In respiratory distress syndrome (RDS), (also called hyaline membrane disease), there is a deficiency of surfactant, which lowers surface tension. Surfactant is a mixture of phospholipids and proteins excreted by the type II pneumocytes of the alveolar epithelium. Surfactant deficiency leads to widespread alveolar collapse and inadequate gas exchange. The more preterm the infant, the higher the incidence of RDS; it is common in infants born before 28 weeks’ gestation and tends to be more severe in boys than girls. Surfactant deficiency is rare at term but may occur in infants of diabetic mothers. The term hyaline membrane disease derives from a proteinaceous exudate seen in the airways on histology. Glucocorticoids, given antenatally to the mother, stimulate fetal surfactant production and are given if preterm delivery is anticipated (see Ch. 9.) The development of surfactant therapy has been a major advance. The preparations are natural, derived from extracts of calf or pig lung. They are instilled directly into the lung via a tracheal tube. Multinational placebo-controlled trials show that surfactant treatment reduces mortality from RDS by about 40%, without increasing the morbidity rate (Fig. 10.11). At delivery or within 4 h of birth, babies with RDS develop clinical signs of: • laboured breathing with chest wall recession (particularly sternal and subcostal indrawing) and nasal flaring • expiratory grunting in order to try to create positive airway pressure during expiration and maintain functional residual capacity The characteristic chest X-ray appearance is shown in Figure 10.12. Treatment with raised ambient oxygen is required, which may need to be supplemented with continuous positive airway pressure (delivered via nasal cannulae) or artificial ventilation via a tracheal tube. The ventilatory requirements need to be adjusted according to the infant’s oxygenation (which is measured continuously), chest wall movements and blood gas analyses. Mechanical ventilation (with intermittent positive pressure ventilation or high-frequency oscillation) may be required. High-flow humidified oxygen therapy, via nasal cannulae, may be used to wean babies from added oxygen therapy. In respiratory distress syndrome, air from the overdistended alveoli may track into the interstitium, resulting in pulmonary interstitial emphysema (PIE). In up to 10% of infants ventilated for RDS, air leaks into the pleural cavity and causes a pneumothorax (Fig. 10.13). When this occurs, the infant’s oxygen requirement usually increases, and the breath sounds and chest movement on the affected side are reduced, although this can be difficult to detect clinically. A pneumothorax may be demonstrated by transillumination with a bright fibreoptic light source applied to the chest wall. A tension pneumothorax is treated by inserting a chest drain. In order to try and prevent pneumothoraces, infants are ventilated with the lowest pressures that provide adequate chest movement and satisfactory blood gases. • they have a large surface area relative to their mass, so there is greater heat loss (related to surface area) than heat generation (related to mass) • their skin is thin and heat permeable, so transepidermal water loss is important in the first week of life • they have little subcutaneous fat for insulation • they are often nursed naked and cannot conserve heat by curling up or generate heat by shivering. There is a neutral temperature range in which an infant’s energy consumption is at a minimum level. In the very immature baby, this neutral temperature is highest during the first few days of life and subsequently declines. The temperature of these small babies is maintained using incubators (Fig. 10.14) or initially with overhead radiant heaters. Incubators also allow ambient humidity to be provided, which reduces transepidermal heat loss.
Neonatal medicine
Hypoxic-ischaemic encephalopathy
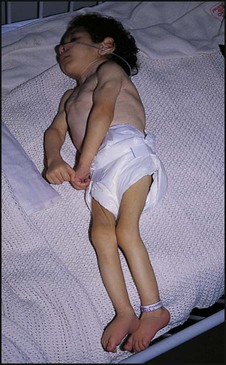
Management
Prognosis
Birth injuries
Soft tissue injuries
Nerve palsies
The preterm infant
Respiratory distress syndrome
Pneumothorax
Temperature control