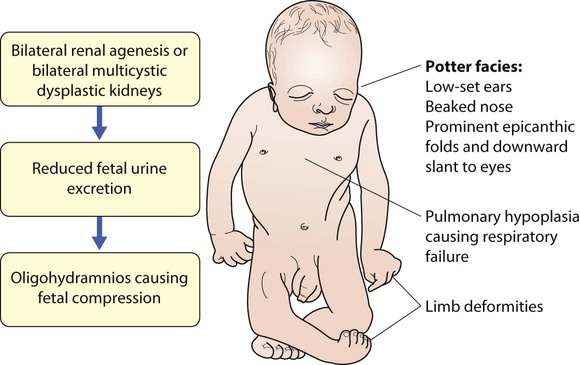
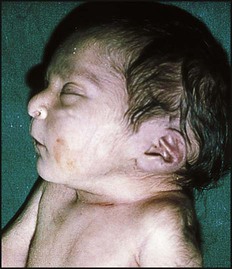
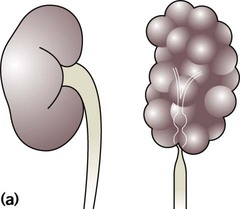
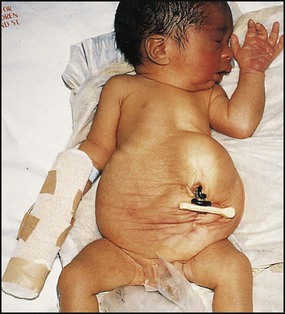
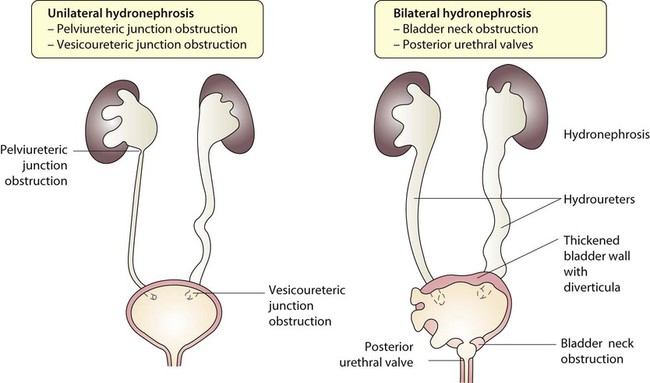
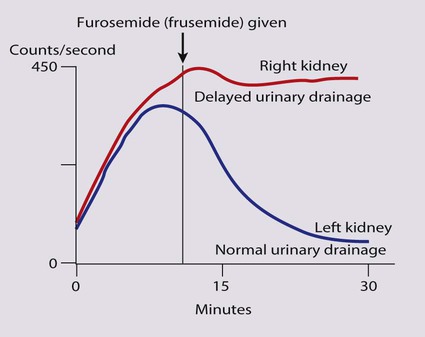
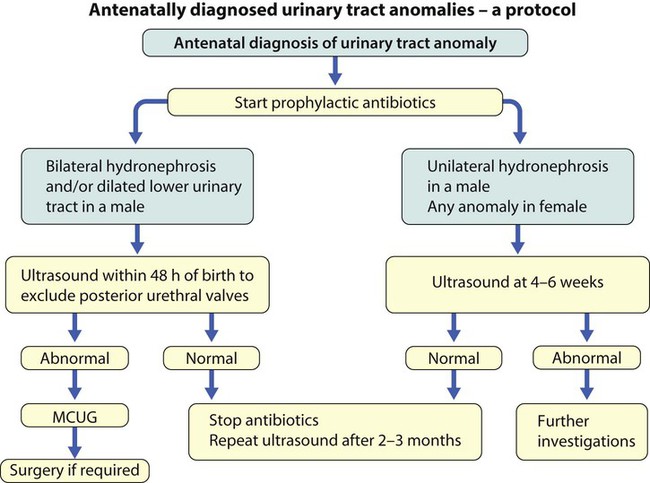
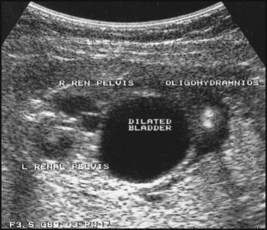
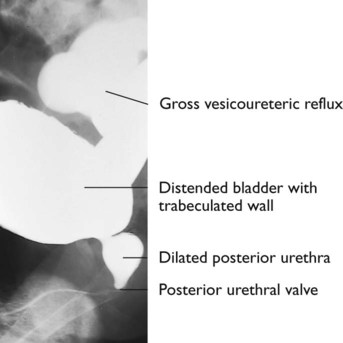
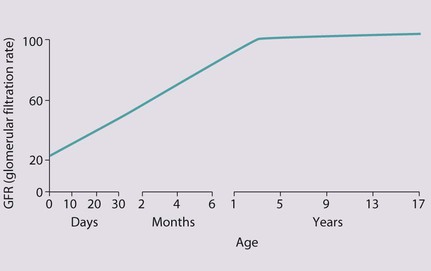
The spectrum of renal disease in children differs from that in adults: • Many structural abnormalities of the kidneys and urinary tract are identified on antenatal ultrasound screening • Urinary tract infection, vesicoureteric reflux and urinary obstruction have the potential to damage the growing kidney • Nephrotic syndrome is usually steroid-sensitive and only rarely leads to chronic renal failure • Chronic renal disorders and the drugs used to treat them may affect growth and development. The glomerular filtration rate (GFR) is low in the newborn infant and is especially low in premature infants; the GFR at 28 weeks’ gestation is only 10% of the term infant. In term infants, the corrected GFR (15–20 ml/min per 1.73 m2) rapidly rises to 1–2 years of age when the adult rate of 80 to 120 ml/min per 1.73 m2 is achieved (Fig. 18.1). The assessment of renal function in children is listed in Table 18.1 and the radiological investigations of the kidneys and urinary tract in Table 18.2. Table 18.1 Assessment of renal function in children Table 18.2 Radiological investigation of the kidneys and urinary tract Standard imaging procedure of the kidneys and urinary tract, provides anatomical assessment but not function. Excellent at visualising urinary tract dilatation, stones and nephrocalcinosis (small, multiple calcium deposits in renal parenchyma) Advantages: non-invasive, mobile Disadvantages: operator-dependent, will not detect all renal scars • be associated with abnormal renal development or function • predispose to postnatal infection • involve urinary obstruction which requires surgical treatment. Absence of both kidneys (renal agenesis) – as amniotic fluid is mainly derived from fetal urine, there is severe oligohydramnios resulting in Potter syndrome (Fig. 18.2a, 18.2b), which is fatal. Multicystic dysplastic kidney (MCDK) – results from the failure of union of the ureteric bud (which forms the ureter, pelvis, calyces and collecting ducts) with the nephrogenic mesenchyme. It is a non-functioning structure with large fluid-filled cysts with no renal tissue and no connection with the bladder (Fig. 18.3). Half will have involuted by 2 years of age, and nephrectomy is indicated only if it remains very large or hypertension develops, but this is rare. Since they produce no urine, Potter syndrome will result if the lesion is bilateral. Other causes of large cystic kidneys are autosomal recessive polycystic kidney disease (ARPKD) (Fig. 18.4), autosomal dominant polycystic kidney disease (ADPKD) (Fig. 18.5) and tuberous sclerosis. In contrast to a multicystic dysplastic kidney, in these disorders some or normal renal function is maintained but both kidneys are always affected. Autosomal dominant polycystic kidney disease has an incidence of 1 in 1000; the main symptoms in childhood are hypertension and haematuria and it causes renal failure in late adulthood. It is associated with several extra-renal features including cysts in liver and pancreas, cerebral aneurysms and mitral valve prolapse. Abnormal caudal migration may result in a pelvic kidney or a horseshoe kidney (Fig. 18.6), when the lower poles are fused in the midline. The abnormal position may predispose to infection or obstruction to urinary drainage. Premature division of the ureteric bud gives rise to a duplex system, which can vary from simply a bifid renal pelvis to complete division with two ureters. These ureters frequently have an abnormal drainage so that the ureter from the lower pole moiety often refluxes, whereas the upper pole ureter may drain ectopically into the urethra or vagina or may prolapse into the bladder (ureterocele) and urine flow may be obstructed (Fig. 18.7). Failure of fusion of the infraumbilical midline structures results in exposed bladder mucosa (bladder extrophy). Absence or severe deficiency of the anterior abdominal wall muscles is frequently associated with a large bladder and dilated ureters (megacystis-megaureters) and cryptorchidism, the absent musculature syndrome (prune-belly syndrome) (Fig. 18.8). Obstruction to urine flow may occur at the pelvi-ureteric or vesicoureteric junction, at the bladder neck (e.g. due to disruption of the nerve supply, neuropathic bladder) or at the posterior urethra in a boy due to mucosal folds or a membrane, known as posterior urethral valves. The consequences of obstruction to urine flow are shown in Figures 18.9a–18.9d. At worst, this results in a dysplastic kidney which is small, poorly functioning and may contain cysts and aberrant embryonic tissue such as cartilage. In the most severe and bilateral cases Potter syndrome is present. Renal dysplasia can also occur in association with severe intrauterine vesicoureteric reflux, in isolation or in certain rare, inherited syndromes affecting multiple systems. An example of a protocol for infants with antenatally diagnosed anomalies is shown in Figure 18.10. Prophylactic antibiotics may be started at birth to try to prevent urinary tract infection, although practice varies between centres. As the newborn kidney has a low GFR, urine flow is low and mild outflow obstruction may not be evident in the first few days of life. The ultrasound scan should therefore be delayed for several weeks. However, bilateral hydronephrosis in a male infant warrants an ultrasound shortly after birth to exclude posterior urethral valves, which always requires urological intervention such as cystoscopic ablation (Case History 18.1). • up to half of patients have a structural abnormality of their urinary tract • pyelonephritis may damage the growing kidney by forming a scar, predisposing to hypertension and to chronic renal failure if the scarring is bilateral. Presentation of UTI varies with age (Box 18.1). In infants, symptoms are non-specific; fever is usually but not always present, and septicaemia may develop rapidly. The classical symptoms of dysuria, frequency and loin pain become more common with increasing age. Serious illness from septicaemia is described in the child with a fever in Chapter 14. Dysuria alone is usually due to cystitis, or vulvitis in girls or balanitis in uncircumcised boys. Symptoms suggestive of a UTI may also occur following sexual abuse. For the child in nappies, urine can be collected by: • A ‘clean-catch’ sample into a waiting clean pot when the nappy is removed. This is the recommended method • An adhesive plastic bag applied to the perineum after careful washing, although there may be contamination from the skin • A urethral catheter if there is urgency in obtaining a sample and no urine has been passed • Suprapubic aspiration (SPA), when a fine needle attached to a syringe is inserted directly into the bladder just above the symphysis pubis under ultrasound guidance; it may be used in severely ill infants requiring urgent diagnosis and treatment, but it is an invasive procedure, and is increasingly replaced by urethral catheter sampling. Ideally, the urine sample should be microscoped to identify organisms and cultured straight away. This is indicated in all infants and children <3 years old with a suspected UTI. If this is not possible, the urine sample should be refrigerated to prevent the overgrowth of contaminating bacteria. Urinary white cells are not a reliable feature of a UTI, as they may lyse during storage and may be present in febrile children without a UTI and in children with balanitis or vulvovaginitis. Dipsticks can be used as a screening test. Urine culture should still be performed unless both leucocyte esterase and nitrite are negative or if the clinical symptoms and dipstick tests do not correlate (Table 18.3). Table 18.3 Methods and interpretation of dipstick testing in children Vesicoureteric reflux (VUR) is a developmental anomaly of the vesicoureteric junctions. The ureters are displaced laterally and enter directly into the bladder rather than at an angle, with a shortened or absent intramural course. Severe cases may be associated with renal dysplasia. It is familial, with a 30–50% chance of occurring in first-degree relatives. It may also occur with bladder pathology, e.g. a neuropathic bladder or urethral obstruction, or temporarily after a UTI. Its severity varies from reflux into the lower end of an undilated ureter during micturition to the severest form with reflux during bladder filling and voiding, with a distended ureter, renal pelvis and clubbed calyces (Fig. 18.12). Mild reflux is unlikely to be of significance, but the more severe degrees of VUR may be associated with intrarenal reflux (IRR), the backflow of urine from the renal pelvis into the papillary collecting ducts; intrarenal reflux is associated with a particularly high risk of renal scarring if UTIs occur. The incidence of renal defects increases with increasing severity of reflux. There is considerable controversy as to whether renal scarring is a congenital abnormality already present in children with reflux and which predisposes to infection or if children with reflux have normal kidneys at birth which are damaged by UTIs and that preventing UTIs in these children prevents scars. Reflux tends to resolve with age especially lower grades of VUR. Reflux with associated ureteric dilatation is important, as: • urine returning to the bladder from the ureters after voiding results in incomplete bladder emptying, which encourages infection • the kidneys may become infected (pyelonephritis), particularly if there is intrarenal reflux • bladder voiding pressure is transmitted to the renal papillae; this may contribute to renal damage if voiding pressures are high.
Kidney and urinary tract disorders
Assessment of the kidneys and urinary tract
Plasma creatinine concentration (PCr)
Main test of renal function. Rises progressively throughout childhood according to height and muscle bulk. May not be outside laboratory ‘normal range’ until renal function has fallen to less than half normal
Estimated glomerular filtration rate (eGFR)
The formula eGFR = k × height (cm) ÷ creatinine (µmol/L) provides estimate of GFR. Better measure of renal function than creatinine and useful to monitor renal function serially in children with renal impairment (k is 40 if creatinine measured using Jaffe method or 30 if measured enzymatically)
Inulin or EDTA glomerular filtration rate
More accurate as clearance from the plasma of substances freely filtered at the glomerulus, and is not secreted or reabsorbed by the tubules. Need for repeated blood tests limits use in children
Creatinine clearance
Requires timed urine collection and blood tests. Rarely done in children as inconvenient and inaccurate
Plasma urea concentration
Increased in renal failure, often before creatinine starts rising, and raised levels may be symptomatic. Urea levels also increased by high protein diet and if in a catabolic state.
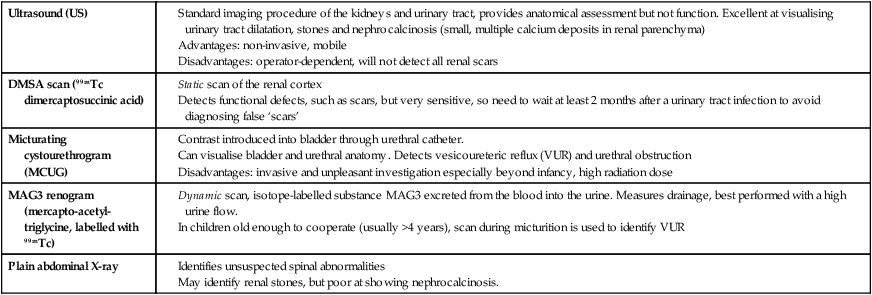
Ultrasound (US)
DMSA scan (99mTc dimercaptosuccinic acid)
Micturating cystourethrogram (MCUG)
MAG3 renogram
(mercapto-acetyl-triglycine, labelled with 99mTc)
Plain abdominal X-ray
Congenital abnormalities
Anomalies detectable on antenatal ultrasound screening
Postnatal management
Urinary tract infection
Clinical features
Collection of samples
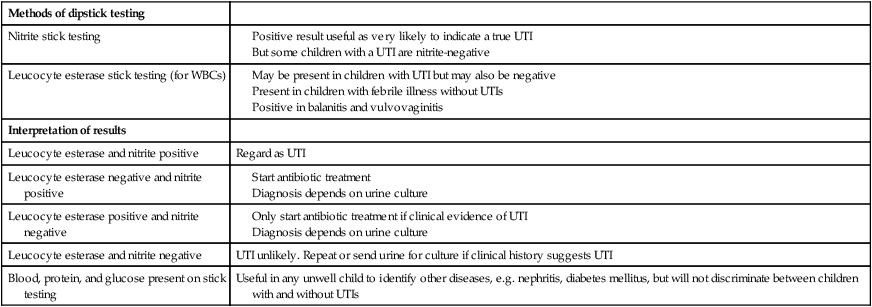
Methods of dipstick testing
Nitrite stick testing
Leucocyte esterase stick testing (for WBCs)
Interpretation of results
Leucocyte esterase and nitrite positive
Regard as UTI
Leucocyte esterase negative and nitrite positive
Leucocyte esterase positive and nitrite negative
Leucocyte esterase and nitrite negative
UTI unlikely. Repeat or send urine for culture if clinical history suggests UTI
Blood, protein, and glucose present on stick testing
Useful in any unwell child to identify other diseases, e.g. nephritis, diabetes mellitus, but will not discriminate between children with and without UTIs
Bacterial and host factors that predispose to infection
Infecting organism
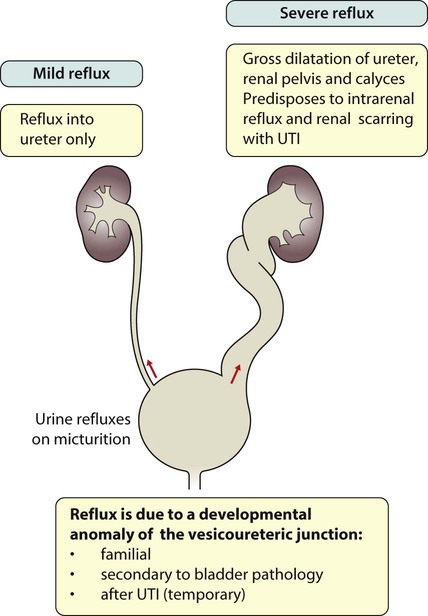
Vesicoureteric reflux
Kidney and urinary tract disorders