Fig. 1
Hector strikes Teucer with a jagged rock in the region of his left clavicle

Fig. 2
Achilles uses his spear to strike Hector in the region of his right clavicle
The physician named William Smellie (1697–1763) was a Scottish-born obstetrician who practiced and taught in London from 1739 to 1759 (Roberts et al. 2010; Fig. 3). He became famous for many things including obstetrical forceps and a special breech delivery technique. Smellie is commonly credited with the earliest known English language description of brachial plexus injury (McGillicuddy 2011). In his book he describes the following events:


Fig. 3
William Smellie (1697–1763)
In the year 1746, about nine o’clock in the morning, I was called by a gentleman who had formerly attended my lectures, to a woman in labour, and found the child’s face presenting… [using forceps for delivery] I pulled gently, moving the head from ear to ear, until it was brought lower down into the pelvis, then …I turned the chin and anterior part of the neck forwards, from the lower part of the ischium to the space below the pubes, so the forehead was at the same time turned from the left ischium to the lower part of the sacrum and coccyx. Lastly, I moved the handles towards the pubes, and delivered the woman of child, whole face was swelled, and whole head was compressed … the long compression had rendered the arms paralyzed for several days, though this misfortune was soon remedied by friction and embrocations. (Smellie 1784)
The large number of cadaveric specimens utilized by Smellie, as well as his contemporary William Hunter (known as the father of modern surgery), has drawn renewed attention in recent years. Theories related both to body snatching schemes and outright murder have been advanced by the New Zealand medical historian Don C. Shelton (2010, 2012).
In 1872 (over 100 years after Smellie’s death), the French physician Guillaume Benjamin Amand Duchenne coined the term “obstetrical palsy” and established its etiology as a neurogenic one (Schmitt et al. 2008; Fig. 4). This “obstetrical” term is now considered obsolete by many, and more accurate terms such as NBPP are preferred (Phua et al. 2012). In 1877 the German physician Wilhelm Heinrich Erb offered an important anatomical breakthrough when he described the C5–C6 junction (aka Erb’s point) as a common area of injury for brachial plexus palsy patients (Watt et al. 2007; Schmitt et al. 2008; Tubbs et al. 2007; Fig. 5). These two physicians have become linked by the hyphenated eponym of Erb-Duchenne brachial plexus palsy (Mehlman 2009).



Fig. 4
Guillaume Benjamin Amand Duchenne (1806–1875)

Fig. 5
Wilhelm Heinrich Erb (1840–1921)
Augusta Klumpke (1859–1927) was born in San Francisco but later moved to Europe where she completed her medical education in Paris and went on to break a major gender barrier when she became the first female intern in Paris (really a residency by modern standards) (Fig. 6; Satran 1974; Shoja and Tubbs 2007; Ulgen et al. 2008). While still a medical student, Klumpke was inspired by the published work of Erb and undertook brachial plexus-focused research of her own (Shoja and Tubbs 2007). In her paper (published in 1885), she elegantly made the case that the oculopupillary phenomenon (aka Horner syndrome or Bernard-Horner syndrome) was indicative of proximal nerve root injury to the lower plexus, what she termed “radicular paralyses” (Ulgen et al. 2008). Her assertion was contrary to prevailing opinion at the time that all brachial plexus injuries gave rise to this ocular finding. During her internship, she married one of her former professors, French neurologist Joseph Jules Dejerine, and thus, literature references often list her as Dejerine-Klumpke (Shoja and Tubbs 2007; Ulgen et al. 2008).


Fig. 6
Augusta Klumpke (1859–1927)
James Warren Sever (1878–1964) (Fig. 7) and Joseph Battiato L’Episcopo (1890–1947) (Fig. 8) contributed important secondary reconstructive principles aimed at improving upper extremity function of NBPP patients (Sever 1916, 1918; L’Episcopo 1934, 1939). Sever focused on surgical release of contracted (dysplastic?) (Nikolaou et al. 2011) structures, while L’Episcopo added muscle transfers aimed at maintaining active external rotation. The work of Sever (1916) can also be pointed to as a watershed moment in the history of NBPP due to its size (36-page monograph) and its comprehensiveness (he reviewed the world’s literature from the mid-1700s on), and it was widely read – as it clearly inspired L’Episcopo and many, many others (Mehlman 2007). These two figures remain linked by the hyphenated eponym: Sever-L’Episcopo procedure.



Fig. 7
James Warren Sever (1878–1964)

Fig. 8
Joseph Battiato L’Episcopo (1890–1947)
Algimantas Otonas Narakas (1927–1993) pioneered brachial plexus surgical repair techniques both before and after the introduction of the surgical microscope (Fig. 9; Egloff 1995; Taleb et al. 2013). He was born in Lithuania but went on to become a Swiss citizen and practice his entire surgical career in Switzerland (Egloff and Bonnard 1995). His eponymical contributions include the Narakas classification (which groups NBPP patients into four disease severity groups based on the number of involved nerve roots) and the Narakas meeting, traditionally referred to as the Narakas Club (recognized as the preeminent every other year brachial plexus meeting on the planet) (Al-Qattan et al. 2009; Palazzi et al. 1999; Taleb et al. 2013).


Fig. 9
Algimantas Otonas Narakas (1927–1993)
Kaiser Wilhelm II
The impact of neonatal brachial plexus palsy on world history has been frequently discussed as it relates to Kaiser Wilhelm II (1859–1941), the notorious Emperor of Germany in World War I (Fig 10; Jacoby 2008). Kaiser Wilhelm II was the son of Princess Victoria (eldest daughter of Queen Victoria) and Kaiser Wilhelm I. The primiparous 19-year-old mother-to-be had been in labor for at least 9 h when Queen Victoria’s personal physician Sir James Clark diagnosed the baby’s breech presentation. Urgent arrangements were made for Professor Eduard Arnold Martin (a prominent German obstetrician) to take over responsibility for the delivery. Martin used chloroform as well as the breech delivery technique described by Smellie nearly 100 years earlier to successfully deliver the baby (Ober 1992). Within several days it was ascertained that the baby’s left arm was abnormally weak, and subsequently it did not grow properly (Jain et al. 2005).


Fig. 10
Kaiser Wilhelm II (1859–1941)
Kaiser Wilhelm II blamed the lifelong disability of his left arm on the failure of the English physician (Sir James Clark) to properly respond during his birth. As fate would have it, there was also an English physician involved with the care of his father, Kaiser Wilhelm I, near the time of his death. This led to Kaiser Wilhelm II stating years later, “An English doctor killed my father, and an English doctor crippled my arm; and all this I owe to my mother who would not have Germans around her” (Jain et al. 2005). This has led some historians to propose that this solidified a deep-seeded hate for the English and thus helped shape world history (Ober 1992).
Epidemiology
Incidence
Early efforts at studying the incidence of NBPP in the United States were limited by the information systems of the day. In addition to this, most reviews were also focused on a single institution or metropolitan area (Foad et al. 2008). For instance, a 1975 publication from the University of California reviewed over 19,000 newborn infants and identified only 11 babies with brachial plexus palsy, yielding an incidence of 0.57 per 1,000 live births (Specht 1975). A ten-year analysis (1972–1982) of data from the Kaiser Foundation Hospital in San Francisco identified 61 babies with birth palsy out of a cohort of over 30,000 deliveries for an incidence of 2.0 per 1,000 live births (Greenwald 1984). At Johns Hopkins an 11-year (1993–2004) review revealed a surprisingly high 5.8 per 1,000 live birth rate (135 palsies among 23,273 births) (Gurewitsch 2006).
A 23-year (1980–2002) review of records at the University of Mississippi produced 85 NBPP cases from over 89,000 vaginal deliveries (incidence = 1.0 per 1,000 live births) (Chauhan et al. 2005). These same authors offered provocative evidence-based extrapolation of their data in that they estimated that the average obstetrician (typically 140 deliveries per yr for ACOG fellows) should encounter NBPP once every 7 years and a permanent plexus injury once every 71 years (Chauhan et al. 2005). A 1999 publication of computerized database analysis of over 300 hospitals across California found an incidence of 1.5 per 1,000 live births (1,611 palsies in over one million deliveries) (Gilbert et al. 1999). The first large US national database study of NBPP included over 17,000 palsy patients from a pool of more than 11 million births, yielding an incidence of 1.5 per 1,000 live births (Foad et al. 2008). Interestingly, 54 % of the babies with NBPP did not have known risk factors (Foad et al. 2008).
Risk Factors
Multiple risk factors have been suggested as predictive of NBPP, but numerous studies have pointed to shoulder dystocia and macrosomia (usually defined as birth weight >4.5 kg) as being exceptionally strong ones (Foad et al. 2008; Mollberg et al. 2005; Chauhan et al. 2005). In an American national database study, shoulder dystocia and macrosomia were associated, respectively, with a 100 times and 14 times higher risk of neonatal plexus palsy (Fig. 11; Foad et al. 2008). It would then seem to simply be necessary to pre-identify macrosomic infants (perhaps by ultrasound) in order to identify those at increased risk for shoulder dystocia and thus at greater risk for NBPP. But in reality ultrasound is an imperfect predictor of macrosomia (Sacks and Chen 2000; Mehta et al. 2005; Goetzinger et al. 2014; Nguyen et al. 2013), macrosomia is an imperfect predictor of shoulder dystocia (Chauhan et al. 2006; Gherman et al. 2006; Alsunnari 2005), and shoulder dystocia is an imperfect predictor of NBPP (Chauhan et al. 2007; Christoffersson et al. 2003).
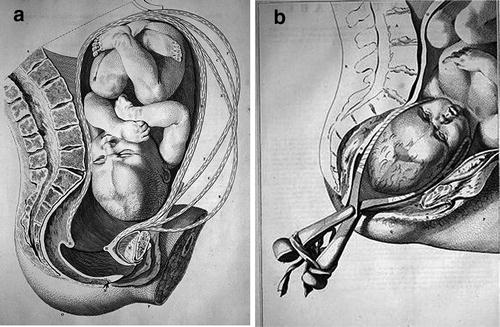

Fig. 11
Shoulder dystocia. (a) Baby higher in the birth canal. (b) Shoulder dystocia has occurred. Smellie’s illustration from 1780, also illustrating application of Smellie forceps
Other contemporary facts regarding shoulder dystocia muddy the water even further. It has been shown that there was a tenfold increase in the rate of shoulder dystocia in recent decades (Dandolu et al. 2005), and surprisingly there are mixed results at best regarding the effectiveness of obstetrical educational programs aimed at managing shoulder dystocia (Walsh 2011; Inglis et al. 2011). Many obstetricians consider the only reliable predictor of shoulder dystocia to be a prior maternal history of shoulder dystocia (Bingham et al. 2010; Ouzounian et al. 2012). On top of all of this, rates of NBPP have remained stubbornly steady over the course of decades despite the above noted increase in shoulder dystocia rates as well as substantial increases in Cesarean section rates in the United States (Walsh et al. 2011; Graham 1997). This has led numerous authors to point to the shortcomings of commonly accepted risk factors (Backe 2008; Gurewitsch 2006; Sandmire and DeMott 2005) and others to assert that one or more known risk factors are present in only a minority (46 %) of newborn babies with NBPP (Foad et al. 2008).
This previous discussion shows that as clear as it is in many cases that traction-related birth trauma seems to be the explanation for the neonatal brachial plexus injury, in other instances, it is equally mysterious as to exactly how or precisely when the injury occurred. This controversy regarding the mechanism of injury is not at all new. James Warren Sever conducted extensive infantile cadaver experiments and concluded:
…that traction and forcible separation of the head and shoulder puts the upper cords, the fifth and sixth cervical roots of the brachial plexus, under dangerous tension. This tension is so great that the two upper cords stand out like violin strings. Any sudden force applied with the head bent to the side and the shoulder held would without question injure these cords … One thing impressed me, and that was the evident vulnerability of the upper cords of the plexus under any degree of traction, and I was surprised that the paralysis was not of much more frequent occurrence. (Sever 1916)
But in Sever’s same 1916 report of 470 NBPP patients, there is a solid 7 % who were born following “apparently normal labors” (Sever 1916). Many others have also traditionally interpreted William Smellie’s 1746 birth palsy description (cited earlier in this chapter) as supportive of an intrauterine etiology.
This provocative issue has been taken on in recent years by multiple authors at a variety of centers (Jennett et al. 1992; Sandmire and DeMott 2000; Gonik et al. 2000). In 1992 Jennett et al. concluded that “the data are strongly suggestive that intrauterine maladaptation may play a role in brachial plexus impairment” (Jennett et al. 1992). Bernard Gonik’s classic paper published in 2000 used mathematical modeling to contrast endogenous forces (uterine and maternal expulsive efforts) to exogenous forces (clinician applied forces), concluding that the endogenous forces were four to nine times greater than clinician-related forces (Gonik et al. 2000). This line of research drew attention to potential differences between difficult and seemingly uncomplicated labors.
Gurewitsch and her colleagues looked at this question from the perspective of plexus palsies that occurred with and without shoulder dystocia and concluded that non-shoulder dystocia-associated NBPP was likely to be a mechanically distinct entity (Gurewitsch 2006). They tracked 13 separate risk factors and found that 11 % of shoulder dystocia-associated brachial plexus palsy patients had no identifiable risk factors as compared to 30 % of non-shoulder dystocia plexus palsy patients. These risk factors ranged from well-known ones such as macrosomia and instrumented delivery to somewhat less well-known risk factors such as long second stage of labor and excessive maternal weight gain (Gurewitsch 2006). It thus becomes increasingly clear that alternate mechanism of injury explanations need to be entertained.
A growing number of authors have drawn attention to intended and unintended outcomes associated with oxytocin augmentation of labor (Wei et al. 2010; Mori et al. 2011; Bugg et al. 2011; Mehlman et al. 2012; Costley and East 2012; Miller 2009; Doyle et al. 2011; Ouzounian et al. 2005). High- and low-dose approaches to oxytocin administration exist, and tachysystole (the term used for uterine hyperstimulation) occurs more frequently with high-dose regimens (Wei et al. 2010). In a case-control study of 52 mothers and their NBPP babies versus 132 mothers and their unaffected babies, oxytocin administration and tachysystole were statistically significant risk factors for NBPP (respective odds ratios of 2.5 and 3.7) (Mehlman et al. 2012). It would seem plausible that a child traversing the birth canal with a leading head and trailing shoulder (or perhaps other positions as well) who experiences significant uterine forces might very well sustain NBPP.
Classification Systems and Outcome Measures
In order to have hope regarding scientific progress in the clinical care of NBPP patients, there must be valid and reliable tools on both the “front end” of the disease (e.g., disease severity measures, age-specific rating scales) and the “back end” of the disease (e.g., health-related quality of life, functional outcomes) (Chang et al. 2013). Validity addresses the issue of whether the instrument (questionnaire, rating scale, etc.) measures what it is intended to measure, and reliability speaks to its ability to yield appropriate reproducible measurements. Collectively these are referred to as psychometric properties. Some of these NBPP tools have yet to undergo formal reliability testing while a number of researchers at different neonatal brachial plexus centers have performed important reliability and validity studies for others. New NBPP-specific instruments also continue to evolve (Ho et al. 2012). This section will focus on the most widely used and most widely studied NBPP tools that are currently available.
The classic NBPP disease severity measure is the Narakas classification. In 1987 Algimantas Narakas proposed that based on physical examination at 2–3 weeks of age, patients could be practically divided into four groups (Narakas 1987). The original classification recognized groups 1 and 2 as classic Erb-Duchenne palsy (C5 and C6) and extended Erb-Duchenne (C5, C6, and C7), while groups 3 and 4 were total plexus palsies without and with the presence of Horner syndrome. The very presence of Horner syndrome (aka Klumpke’s sign as she was the first to describe the association between brachial plexus injury and these oculopupillary findings) has been shown to have independent unfavorable prognostic value (see Fig. 12 baby photo) (Al-Qattan et al. 2000). These Narakas groupings have been shown to have prognostic power, with dramatically lower full recovery rates for Narakas 3 and 4 patients (Narakas 1987; Sibinski and Synder 2007; Foad et al. 2009).


Fig. 12
Clinical photo of an infant with left Narakas 4 neonatal brachial plexus palsy
Al-Qattan and his Saudi Arabian colleagues have recently reviewed over 500 of their NBPP patients and produced a useful modification of the Narakas classification (see Table 1) (Al-Qattan et al. 2009). They found that Narakas group 2 patients who recovered their wrist extension early (between 2 and 3 weeks of age and 2 m of age) had better rates of spontaneous recovery than those who did not (Al-Qattan et al. 2009). For instance, 76 % of Narakas 2-A patients recovered shoulder abduction, while only 18 % of Narakas 2-B patients did the same. It can be argued that the validity of the Narakas classification system (and now the modified Narakas classification) is self-apparent, but formal reliability studies have yet to be published.
Table 1
Modified Narakas classification
Group | Name | Roots | Key findings |
|---|---|---|---|
1 | Typical Erb-Duchenne palsy | C5, C6 | Wrist extension often weak |
Hand not affected | |||
2 | Extended Erb-Duchenne palsy | C5, C6, C7 | Same as 1 + active elbow extension not as strong and definite wrist drop |
2-A = early recovery wrist extension against gravity (2–3 weeks to 2 months) | |||
2-B = no early recovery wrist extension against gravity (>2 months) | |||
3 | Total palsy without Horner sign | C5, C6, C7, C8, T1 | Wrist flexed, hand tightly closed |
4 | Total palsy with Horner sign | C5, C6, C7, C8, T1 | Horner sign indicates preganglionic T1 injury |
Disease severity is also commonly assessed by the Toronto Test Score , a tool aimed at assessing children less than 1 year of age. This scale was introduced in 1994 by brachial plexus specialists at the Hospital for Sick Children in Toronto, Ontario (Michelow 1994). The Toronto Test Score combines physical examination-derived data from five distinct muscle groups, one of which is elbow flexion (see Table 2). Prior to the Toronto Test Score, many brachial plexus surgeons used just the presence or absence of elbow flexion at a particular age (e.g., 3 months of age) as their main criterion for recommending nerve reconstruction surgery. The Toronto Test Score effectively changed this to multidimensional decision making. The original article showed that the Toronto Test Score at 3 months of age correctly predicted the child’s clinical status at 1 year of age 95 % of the time, while similar use of only elbow flexion was predictive in only 13 % of cases (Michelow 1994).
Table 2
Toronto Test Score
Observationa | Grade | Score | Motion | Sub-score |
|---|---|---|---|---|
No joint movement | 0 | 0 | THUMB EXT | – |
Flicker of movement | 0+ | 0.3 | WRIST EXT | – |
Less than half range | 1− | 0.6 | ELBOW EXT | – |
Half range of movement | 1 | 1.0 | ELBOW FLEX | – |
More than half range | 1+
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|