and Spencer W. Beasley2
(1)
Department of Urology, Royal Children’s Hospital, Melbourne, Australia
(2)
Paediatric Surgery Department Otago, University Christchurch Hospital, Christchurch, New Zealand
Abstract
The common causes are described, and how bowel obstruction presents. Then, there is a detailed section on the physical examination.
Obstruction of the bowel at birth is a relatively common abnormality and has a large number of possible causes, most of which are rare individually (Table 21.1). The common forms of obstruction in the neonatal period are Hirschsprung disease and necrotizing enterocolitis. Less frequent conditions include small bowel atresia, either of the ileum or jejunum; malrotation or incomplete rotation of the bowel, with supervening volvulus (twisting of the bowel); duodenal atresia or stenosis; various abnormalities of the anorectal region, including imperforate anus; meconium ileus (as the first manifestation of cystic fibrosis); and perforation of the bowel, either prenatally (causing a sterile meconium peritonitis) or postnatally (causing septic peritonitis).
Table 21.1
Causes of neonatal bowel obstruction
Disease | Frequency |
|---|---|
Hirschsprung disease | More common |
Necrotizing enterocolitis | |
Small bowel atresia | Less common |
Malrotation with volvulus | |
Duodenal atresia/stenosis | |
Imperforate anus | |
Meconium ileus | Uncommon |
Prenatal perforation (meconium peritonitis) |
The pathological processes which lead to bowel obstruction at birth can be divided into three groups: an intrinsic structural abnormality of the bowel; an atresia secondary to occlusion of the blood vessels in utero; and a functional obstruction caused by inflammation, defective innervation or increased viscosity of the contents (Table 21.2). Such a classification has some use in the practical assessment since the symptoms and signs vary with the three groups, and the groups carry different prognostic implications for the baby.
Table 21.2
Causes of neonatal bowel obstruction: pathology
Pathogenesis | Disease |
|---|---|
Failure of gut development | Oesophageal atresia |
Failure of gut canalization | Duodenal atresia |
Ischaemic involution (thromboembolus, volvulus, intussusception) | Small bowel atresia |
Functional obstruction | |
Inflammation | Necrotizing enterocolitis |
Defective nerves | Hirschsprung disease |
Meconium viscosity | Meconium ileus (cystic fibrosis) |
The Causes of Bowel Obstruction
Hirschsprung Disease
The ganglion cells of the intramural plexuses in the bowel control the coordination of contraction and relaxation of normal peristalsis. If these ganglion cells are missing, the bowel appears normal macroscopically, but it is obstructed functionally because of lack of coordinated peristalsis. The degree of resting contractility in the ‘aganglionic’ segment of bowel is variable, and it may be atonic or spastic. Meconium is unable to pass through the aperistaltic segment and leads to a functional obstruction with secondary proximal dilatation. The dilatation occurs in the normally innervated proximal bowel and, in the past, led to the term ‘congenital megacolon’ (Fig. 21.1). The length of colon affected by this deficiency of ganglion cells is variable but in most patients extends from the anus proximally as far as the rectosigmoid junction. Pathological confirmation of Hirschsprung disease is obtained when biopsy of the mucosa just inside the anal canal shows absence of ganglion cells in the submucous (and myenteric) plexus and overgrowth of presynaptic neurofibrils in the mucosa and submucosa (Fig. 21.2). The potential for overgrowth of pathogenic organisms in the dilated and obstructed proximal colon to produce a fulminating enterocolitis, with rapid progression to generalized sepsis and death, makes Hirschsprung disease an important condition to diagnose early.

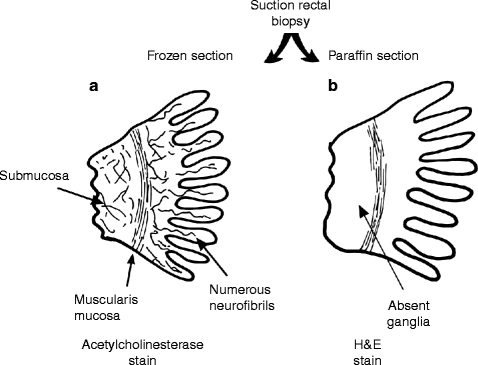

Fig. 21.1
Hirschsprung disease

Fig. 21.2
Pathological diagnosis of Hirschsprung disease from a suction rectal biopsy stained with acetylcholinesterase (a) or H & E (b). Acetylcholinesterase staining reveals numerous extrinsic autonomic neurofibrils ramifying in the submucosa and lamina propria (the extrinsic nerves overgrow because they cannot find an intrinsic nerve to form a synapse). H & E shows that the intrinsic enteric nerves, known as ganglion cells, are absent from the rectal wall
Necrotizing Enterocolitis
Necrotizing enterocolitis is a serious, acquired abnormality of the bowel which appears to be caused by mucosal hypoxia and ischaemia in association with bacterial invasion of the gut wall by necrotizing and gas-forming organisms (Fig. 21.3). Conditions which produce perinatal stress (e.g. prematurity, obstetric complications, sepsis, cyanotic heart disease and metabolic disturbances) predispose to its development. The ischaemic mucosa is colonized by certain pathogenic bacteria which include strains of Clostridium. These organisms multiply within the layers of the bowel wall and produce large gas bubbles known as ‘pneumatosis intestinalis’ or ‘intramural gas’. Further progression of the infection and ischaemia produces gangrene and perforation of the bowel, which leads rapidly to generalized sepsis and peritonitis.


Fig. 21.3
The pathogenesis of necrotizing enterocolitis
Small Bowel Atresia
In a few patients, ileal or jejunal atresia is caused by an intrinsic abnormality in the development of the bowel, but more frequently it is believed to result from infarction of the bowel in utero. It is possible that, in the fetus, the cause of infarction is vascular occlusion of a segmental mesenteric vessel from thrombosis or an embolus.
In addition, mechanical mishaps, such as twisting (volvulus) of the bowel or intussusception, can lead to infarction and subsequent atresia. Vascular or mechanical abnormalities causing perforation also may produce an atresia from scarring at the site of perforation (Fig. 21.4).


Fig. 21.4
The pathogenesis of small bowel atresia from vessel occlusion (a), fetal intussusception (b) or fetal volvulus (c)
Malrotation with Volvulus
In the early embryo, the developing midgut (which will form the small bowel and the right colon) elongates in the space created by the developing umbilical cord. Progressive narrowing of the umbilical ring accompanies the acquisition of the three-dimensional structure of the embryo and enlargement of the abdominal cavity: with this process, the right colon and small bowel return to the abdominal cavity at about 10 weeks of gestation.
As the bowel continues to elongate on return to the abdomen, it undergoes rotation and fixation to the posterior abdominal wall such that its mesentery, which contains the superior mesenteric vessels, is attached along a wide base from the duodenojejunal flexure to the caecum. The colon is fixed in each flank, and the transverse colon is anchored to the stomach by the gastrocolic ligament and greater omentum. When rotation is incomplete or abnormal, the bowel remains unfixed to the posterior abdominal wall (Fig. 21.5), which predisposes to twisting.


Fig. 21.5
A comparison of normal gut fixation and rotation (a) with malrotation (b) and malrotation with secondary volvulus (c)
In the common form of malrotation, the caecum is found just to the left of the midline at the base of the superior mesenteric artery. It has undergone 180° rotation from its original caudal position, but has failed to cross the midline and descend into the right iliac fossa. In this position, the caecum may be fixed to the right lateral abdominal wall and subhepatic region by fibrous bands of condensed peritoneum which cross the non-rotated duodenum (‘Ladd’s bands).
This anatomical arrangement predisposes to twisting of the whole of the midgut on its narrow mesenteric base to cause duodenal obstruction, with or without midgut ischaemia. Bowel malrotation with volvulus is an extremely dangerous condition because it may lead to infarction of the entire midgut – a potentially fatal complication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


