Fig. 7.1
Bubble NCPAP. (A) Respiratory inductance plethysmography abdominal and chest bands; (B) esophageal balloon connected to pressure transducer. (A, B) are being used for pulmonary mechanics measurements in this case. (C) Nasal prongs, (D) manometer, (E) oxygen blender with flowmeter, (F) heated humidifier, (G) inspiratory tubing, (H) expiratory tubing, (I) underwater bubble chamber (J Perinatology 2005 vol 25 p. 454, with permission)
7.2.2 Variable Flow
Variable-flow NCPAP devices change CPAP pressure by adjustments in the flow rate. Variable-flow devices are for the most part freestanding machines that employ a special nosepiece. The nosepiece acts to stabilize mean airway pressure by entraining gas at the inspiratory jets and reduce work of breathing by the Coanda effect, whereby exhalation “flips” the inspiratory gas flow along the path of least resistance – the curved surface of the expiratory pathway (Moa et al. 1988; Pandit et al. 2001) (Fig. 7.2). As of this writing the most commonly employed variable-flow device is the Infant Flow® system (CareFusion, Inc., Yorba Linda, CA) (Fig. 7.3); another very similar device is the Arabella® (Hamilton Medical, Reno, NV). The Benveniste gas-jet valve CPAP device is used predominantly in Scandinavia and is essentially a variable-flow CPAP (Benvieniste et al. 1976). The device consists of a straight nozzle and a curved tube that are positioned coaxially with a ring. CPAP level is changed by increasing or decreasing flow. Pressure generated should be measured with an accurate manometer.
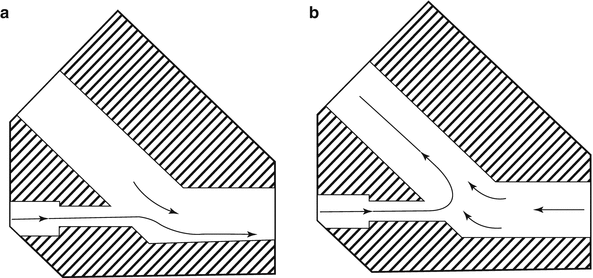
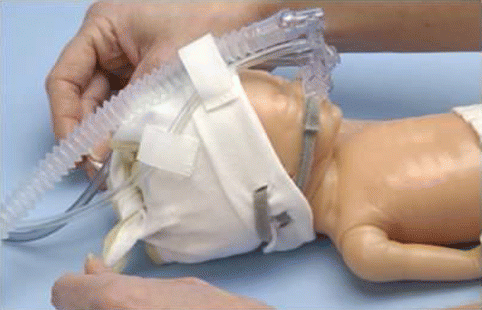

Fig. 7.2
Variable-flow NCPAP generator. (a) Inhalation: jet towards inspiratory branch. (b) Exhalation: jet flow diverted down expiratory branch (Neonatal Intensive Care vol 13 #5 Sep 2000, with permission)

Fig. 7.3
Infant Flow NCPAP generator applied to an infant model (With permission, CareFusion, Inc.)
7.2.3 Bi-level CPAP
Bi-level CPAP provides CPAP at two levels instead of one. The device cycles between levels with a rate and time at the higher level that are operator dependent. The bi-level device available at this writing is SiPAP® (CareFusion, Inc., Yorba Linda, CA) (Fig. 7.4), which functions in the same way as the Infant Flow® system and uses the same special nosepiece. The SiPAP-TR®, available outside of the USA, can be equipped with a Graseby capsule for synchronization of the initiation of the higher CPAP level with the infant’s breathing.


Fig. 7.4
Bi-level NCPAP device (SiPAP, with permission, CareFusion, Inc.)
7.2.4 Nasal Intermittent Positive Pressure Ventilation (NIPPV)
NIPPV uses a non-intubated NCPAP device to provide positive pressure ventilation. NIPPV is discussed elsewhere in this book and will not be included in this chapter.
7.3 Assessing the NCPAP Data
Studies of NCPAP have been varied, extend back over many years, and include a variety of study designs. Additionally, NCPAP is employed using a variety of devices and under a variety of circumstances. It is thus extremely difficult to assess the existing NCPAP studies. We will attempt to do so by discussing the following somewhat arbitrary but nonetheless useful general categories of studies:
1.
NCPAP used at resuscitation (surfactant not part of the study group assignment)
2.
NCPAP used early or prophylactically for respiratory distress (surfactant again not part of the study group assignment)
3.
NCPAP used in conjunction with exogenous surfactant
4.
NCPAP used for extubation
5.
NCPAP used for apnea of prematurity
6.
Comparison of NCPAP devices
To provide an evidence-based approach to assessing the NCPAP data in these categories, we will use the following classification of evidence:
1. Level I | Systematic review of randomized controlled trials |
2. Level II | Randomized controlled trial |
3. Level III | Cohort study |
4. Level IV | Case–control study |
5. Level V | Case series or historical controls |
6. Level VI | Studies completed prior to 1990 |
The inclusion of Level VI, studies completed prior to 1990, is important for respiratory therapies in neonates, as in 1990 exogenous surfactant use was approved by the FDA and its use became commonplace. Exogenous surfactant had a dramatic effect on morbidity and mortality of the preterm infant. About this time, as well, began dramatic differences in how infants were ventilated, including high-frequency oscillatory and jet ventilation, synchronized conventional ventilation, and attention to prevention of volutrauma and barotrauma. Additionally, use of antenatal steroids became more common. Studies done prior to this time may not be relevant to today.
We also will not discuss every article ever published on the topic of NCPAP. Articles included in meta-analysis will in general not be individually discussed. We hope nonetheless to present the articles that will be most helpful and representative of the current literature as of this writing and that this approach will give the reader an idea of the state of the art regarding NCPAP in neonates. Also, in the following Tables, note that “Favors” will imply that some benefit was found.
7.3.1 NCPAP Used at Resuscitation
There is no question that a well-done meta-analysis of randomized controlled trials is usually the highest level of available evidence. However, there are several mitigating factors, including the publication dates of included trials. Several Cochrane reviews of NCPAP use have been published, but many are of limited value today due to inclusion of trials done in the 1970s and 1980s.
Use of positive pressure during resuscitation makes good physiologic sense, as fluid must be absorbed from the alveolar space and alveolar units that may be atelectatic must be opened. Yet until very recently no randomized trials addressed this topic. Use of positive end-expiratory pressure (PEEP) during positive pressure ventilation at resuscitation still has not been sufficiently studied, even today (O’Donnell et al. 2004). Upton and Milner reported a case series of 30 babies studied in the late 1980s and suggested that use of positive end-expiratory pressure (PEEP) during resuscitation might be useful in establishing functional residual capacity (FRC) (Upton and Milner 1991). Lindner et al. reported a retrospective cohort study with historical controls, comparing immediate intubation (control, 1994, n = 56) with immediate NCPAP (1996, n = 67) in extremely low birth weight (ELBW) infants. They found a reduction in need for intubation in the latter group (25 %) (Lindner et al. 1999).
Several more recent studies are of note. In 2004, Finer et al. reported a study of 104 ELBW infants randomized to receive either CPAP/PEEP or not, using a neonatal T-piece resuscitator (NeoPuff Infant Resuscitator, Fisher-Paykel, Auckland, New Zealand). In this pilot study, CPAP/PEEP in the delivery room did not affect the need for intubation, either in the delivery room or in the NICU (Finer et al. 2004). In a study reported in 2007, te Pas and Walther described a randomized trial of 207 infants <33 weeks gestation. Infants received either bag and mask ventilation with minimal PEEP or a sustained inflation then NCPAP, using the NeoPuff. These infants were considerably larger than those reported by Finer (mean birth weight about 1,300 g vs. about 775 g). In these larger infants, 37 % of the NCPAP babies required intubation by 72 h and 51 % of the bag- and mask-ventilated babies (p = 0.04) (te Pas and Walther 2007).
In a large multicenter study, Morley et al. randomized 610 infants of from 25 to 28 weeks gestation to CPAP or intubation at 5 min of life. At 28 days, fewer infants were on oxygen and fewer infants required ventilation in the CPAP group. However, the rate of death or BPD at 36 weeks did not differ between groups, and the incidence of pneumothoraces in the CPAP group was 9 % vs. 3 % in the intubation group (Morley et al. 2008).
Tapia et al. studied 256 infants who weighed 800–1500 g and were breathing spontaneously at birth. Infants were randomized to NCPAP or oxyhood/nasal cannula; subsequent surfactant was given according to preset criteria. Those in the NCPAP group were returned to NCPAP after surfactant, the others were continued on mechanical ventilation. Those in the NCPAP group had reduced need for surfactant therapy and mechanical ventilation.
7.3.2 NCPAP Used Early or Prophylactically for Respiratory Distress
The meta-analysis by Ho et al. of early CPAP vs. delayed CPAP includes only studies completed prior to 1990 (Ho et al. 2010). Thus, though the analysis found subsequent use of mechanical ventilation was reduced with early CPAP, the relevance of this finding to current practice is unknown and we will consider this analysis to be of historical interest (Level VI evidence). Avery et al., in a cohort study of eight centers published in 1987, found a decrease in BPD in one center that used early NCPAP (Avery et al. 1987). In another cohort trial including the years 1988–1993, Jonsson et al. found that only one-third of infants treated with early NCPAP subsequently required mechanical ventilation (Jonsson et al. 1997).
A 2005 meta-analysis by Subramaniam et al. assesses the similar question of prophylactic NCPAP, prior to any signs of respiratory distress. The authors conclude there is insufficient evidence to address this question (Subramaniam et al. 2005). Only two trials are included, one of which was completed prior to 1990. The other trial, published by Sandri et al. in 2004, found no benefit to prophylactic NCPAP (Sandri et al. 2004). The primary endpoint in this trial was the need for exogenous surfactant.
In 2008, Ho et al. updated their meta-analysis on CPAP for respiratory distress and included continuous negative extrathoracic pressure (CNEP) as well as positive pressure. Again, most of the studies were published well prior to 1990, though two of the included studies were completed after 1990. Buckmaster et al. randomly assigned 300 infants, all >30 weeks gestation and at non-tertiary centers, to either NCPAP or oxygen by headbox. Fewer infants on NCPAP required transfer or failed therapy; more NCPAP infants suffered pneumothorax (Buckmaster et al. 2007). The second trial, by Samuels et al., evaluated CNEP vs. headbox oxygen. Surviving infants on CNEP required fewer days on oxygen, 20.5 vs. 38.9. Mortality was not significantly different (Samuels et al. 1996). The meta-analysis, which includes the studies by Buckmaster and Samuels, concluded that continuous distending pressure reduces respiratory failure and mortality, but increases pneumothorax (Ho et al. 2008).
7.3.3 NCPAP Used in Conjunction with Exogenous Surfactant
Interest in early NCPAP use combined with selective surfactant administration as a way to avoid prolonged mechanical ventilation has resulted in multiple recent studies and meta-analyses. In 1994 Verder et al. reported a study in which 68 infants were randomized to either NCPAP+surfactant, or NCPAP alone (Verder et al. 1994) Surfactant was given by what has come to be known as the INSURE technique (INtubate, give SURfactant, and Extubate). The infants were larger than in most subsequent studies, with mean birth weight of >1300 g. The authors found that infants in the NCPAP+surfactant group had reduced need for mechanical ventilation (MV) (43 % vs 85 %; p = 0.003). In a study of 60 much smaller and more immature infants, mean birth weight of about 950 g, Verder et al. examined whether infants requiring NCPAP would fare better with early surfactant therapy (oxygen requirements 37–55 %) or later treatment (oxygen requirements 57–77 %). Infants in the early treatment group had a greatly reduced incidence of death or need for mechanical ventilation (21 % vs. 63 %) (Verder et al. 1999).
Tooley and Dyke randomized 42 infants of 25–28 weeks gestation to early surfactant and extubation to NCPAP vs. early surfactant and MV. By 72 h of age, significantly fewer infants were intubated in the NCPAP group (47 % vs. 81 %) (Tooley and Dyke 2003).
A meta-analysis addressing the question of early surfactant followedby NCPAP vs. continued MV was published in updated form by Stevens et al. in 2007. These reviewers concluded that early exogenous surfactant with extubation to NCPAP, when compared to later, selective exogenous surfactant use with continued MV, is associated with less MV need, lower BPD incidence, and fewer air leaks (Stevens et al. 2007).
Kribs et al. reported in an observational study several cohorts of infants treated from 2000 to 2004. During this time, early NCPAP and early surfactant therapy use became more and more common, and in addition survival increased and BPD rates decreased (Kribbs et al. 2008).
A large study from Columbia by Rojas et al. was reported in 2009. In this randomized trial 279 infants born between 27 and 31 weeks gestation and with evidence of respiratory distress were randomly assigned to early NCPAP/surfactant or NCPAP alone. The primary outcome was the need for subsequent mechanical ventilation. Criteria for MV were predefined. The need for MV was 26 % in the NCPAP/surfactant group vs. 39 % in the NCPAP group (RR = 0.69; 95 % CI 0.49–0.97). Additionally, air leak was less in the NCPAP/surfactant group (2 % vs. 9 %; RR = 0.25; 95 % CI 0.07–0.85) (Rojas et al. 2009).
Summary Table: use of NCPAP plus surfactant
Study | Level of evidence | Favors CPAP + early surf | Neutral | Favors CPAP alone, MV, or late surf |
|---|---|---|---|---|
Verder et al. (1994) | II | X | ||
Verder et al. (1999) | II | X | ||
Tooley and Dyke (2003) | II | X | ||
Stevens et al. (2007) | I | X | ||
Kribbs et al. (2008) | III | X | ||
Rojas et al. (2009) | II | X | ||
Sandri et al. (2010) | II | X | ||
SUPPORT (2010) | II | X | ||
Dunn et al. (2011) | II | X | ||
Rojas-Reyes et al. (2012) | I | X |
Several recent studies have addressed whether prophylactic surfactant is superior to NCPAP with a selective administration of surfactant. In 2009, Sandri et al. reported a randomized comparison of these interventions in 208 infants between 25–28 weeks’ gestation. No differences in need for MV, death, or major morbidities were found between groups (Sandri et al. 2009). In the largest study to date, the SUPPORT Study Group performed a randomized, multicenter trial using a factorial design toassess early CPAP (delivery room) vs. intubation/surfactant (within 1 h of birth) and target ranges of oxygen saturation in extremely preterm infants (those born between 24 weeks 0 days and 27 weeks 6 days). A total of 1,316 infants were enrolled. Death or BPD (defined as oxygen requirement at 36 weeks) was not different between groups. Some secondary outcomes favored the NCPAP group: requirement for intubation, postnatal use of corticosteroids for BPD, days of mechanical ventilation, and mechanical ventilation by day 7 (SUPPORT Study Group 2010).
The Vermont Oxford Network studied three initial management strategies in infants from 26 0/7 to 29 6/7 weeks: prophylactic surfactant followed by mechanical ventilation, prophylactic surfactant with extubation to NCPAP, and initial NCPAP with selective administration of surfactant (Dunn et al. 2011). This study enrolled 648 infants but closed prior to attaining the required sample size of 876. No differences in the primary outcome measure of death or BPD were found, but fewer infants in the NCPAP group required intubation or surfactant.
None of the above studies found a reduction in BPD with use of NCPAP. However, two things are important to note: one, no study has found an increase in BPD with NCPAP/selective surfactant; and two, a recent meta-analysis has found that in studies with routine application of NCPAP, the combined outcome variable of death/BPD favors early NCPAP with selective surfactant use over prophylactic surfactant (RR = 1.12; 95 % CI 1.02–1.24) (Rojas-Reyes et al. 2012).
7.3.4 NCPAP for Extubation
Davis and Henderson-Smart reported a meta-analysis of NCPAP following extubation. Eight of the nine included trials were published after 1990 (Davis and Henderson-Smart 2003). The conclusion of this meta-analysis (assessed as up to date in 2009) was that NCPAP is in fact effective in preventing failure of extubation in preterm infants.
One study, by Robertson and Hamilton, was not included in the meta-analysis because of a somewhat different study design, assessing NCPAP with a weaning regimen if tolerated compared to hood oxygen with an escalation regimen if needed. In this randomized study of 58 infants between 24 and 32 weeks gestation, no differences in extubation success were noted (Robertson and Hamilton 1998).




Summary Table: NCPAP for extubation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


