James W. Van Hook, MD
• Percutaneous Coronary Intervention
• Coronary Artery Bypass Graft Surgery
1. Organic Nitrates (Nitroglycerin: Category B; Isosorbide Dinitrate: Category C)
3. Beta-Adrenergic Blocking Agents (Category C)
4. Calcium Channel Blockers (Category C)
7. Hydroxymethylglutaryl-Coenzyme A Reductase Inhibitors (Category X)
10. Thienopyridine Derivatives (Category B)
INTRODUCTION
Women of reproductive age are usually regarded as young and healthy individuals with a decreased risk of developing a serious illness. However, the physiologic changes that occur during pregnancy are demanding on the cardiovascular system. Total cardiac output increases by about 50% from a combination of increased blood volume and pulse along with a decrease in peripheral resistance. These changes can place a significant stress on a normal heart and can become dangerous to individuals with underlying cardiac disorders. An estimated 0.4% to 4.1% of all pregnancies are complicated by cardiovascular diseases, and the number of patients who develop cardiac problems during pregnancy is increasing.1 Acute myocardial infarction (MI) although a more rare, but possibly lethal event during pregnancy, delivery, or the peripartum period, can occur in previously asymptomatic women who are experiencing the cardiac stress associated with normal pregnancy. Maternal mortality following MI in pregnancy develops secondary to different etiologies such as atherosclerosis, coronary vasospasm, thrombosis, and coronary dissection. Although the number of such patients presenting to the individual physician is small, knowledge of the risks associated with MI during pregnancy and its management are of pivotal importance.
This chapter will focus on acute MI in pregnancy with an emphasis on diagnosis and disease management. The occurrence of the disease during pregnancy, unlike in a nonpregnant patient, requires special consideration to the fact that all measures concern not only the mother but also the fetus. Therefore, the optimum treatment of both must be targeted.
EPIDEMIOLOGY
Incidence
First described by Katz in 1922, the frequency of MI in pregnancy has been increasing in parallel with the growing number of pregnancies in women at older ages. With the continuing trend of childbearing at older ages and advances in reproductive technology enabling older women to conceive, it may be expected that the incidence of MI in pregnancy will continue to increase.2–4 A recent publication reviewing documented cases of pregnancy-associated MI between 1995 and 2005 showed patient ages ranged from 19 to 44 years, but that the majority of cases (72%) occurred in patients who were older than 30 years. In addition, the review noted that MI occurred mostly (78%) in the anterior cardiac wall.5 Ladner et al. in 2005 reviewed hospital discharge records for deliveries in California between 1991 and 2000 and reported an incidence of 1 MI in 35,700 deliveries or 2.8 in 100,000 deliveries.6 Subsequently, James et al. published a population-based study in the United States looking at the Nationwide Inpatient Sample for the years 2000 to 2002 that estimated the incidence of pregnancy-related MI to be 6.2 per 100,000 deliveries.7 The higher incidence reported by James et al. either reflects improvements in diagnostic capabilities or an increasing trend in the number of cases as the pregnancy cohort grows older. This would parallel similar findings reported by Ladner et al. who reported an increase in the incidence of MI in pregnancy over the 10-year study period (1 in 24,000 in the final year compared with 1 in 73,400 initially). Although MI has been reported at all stages of pregnancy and postpartum, it occurs more commonly in the third trimester of pregnancy and in multigravidas.5
Risk Factors
In women of childbearing age, pregnancy alone increases the risk of myocardial ischemia and an acute MI three- to fourfold.5–7 In contrast to ischemic disease found in the older population, peripartum myocardial ischemia is a different entity, as the underlying cause is commonly not atherosclerosis (even though atherosclerosis is still the most common cause during pregnancy).8 The associated pattern of coronary artery disease consists of stenosis (40%), dissection (27%), normal (13%), thrombus (8%), and spasm (2%) or embolus (2%).5,6,9 An additional study has posited that some cases of peripartum MI may be immune mediated in response to a fetal antigen.9 Therefore, the risk factors for an acute MI in pregnancy encompass not only the common cardiovascular risk factors seen in the general population but also risk factors associated with the pregnant state. Ladner et al. identified advanced maternal age, preeclampsia, and eclampsia as independent risk factors for pregnancy-related acute MI. James et al. also found thrombophilia, transfusion, and postpartum infections to be significant risk predictors for acute MI.6,7 Coronary atherosclerotic lesions have been reported in only 20 to 43% of pregnancy-related acute MI cases.4,5 Common risk factors for these lesions include a sedentary lifestyle, a family history of atherosclerotic disease, obesity, smoking, diabetes, dyslipidemia, hypertension, and the use of oral contraceptives.10 Therefore, the increasing rates of maternal obesity, smoking, diabetes mellitus, and hypertension in addition to increasing maternal age are likely contributing to the rise in frequency of pregnancy associated MI.
Spontaneous coronary artery dissection (SCAD) is observed in 15% of cases and is often associated with the hemodynamic stress experienced during labor. It is most often encountered in fairly young otherwise healthy women. In a comprehensive literature review of 119 reported cases, Sheikh and O’Sullivan elucidated that 76 (64%) did not have any risk factors; 7 (6%) had multiple risk factors, while risk factors in others included antiphospholipid syndrome in 2 cases (1.68%), fibromuscular dysplasia in 1 (0.84%), positive family history of ischemic heart disease in 6 (5%), hypercholesterolemia in 2 (1.68%), hypertension in 7 (5.88%), smoking in 11 (9%), oral contraceptives in 1 (0.84%), and unknown risk factors in 6 women (5%).11 There is also an increased risk of SCAD in patients with advancing age and multiparity.12 Approximately 30% of the reported cases occur late in pregnancy (near term) or within 3 months postpartum. It most commonly affects the left main coronary artery, the left anterior descending artery, or both.13–16 SCAD may cause extensive ischemia, which can precipitate severe hemodynamic compromise coinciding with a high risk of maternal and fetal adverse outcomes.17 Especially high levels of estrogens are thought to change the normal arterial wall architecture, resulting in susceptibility to spontaneous dissections. These changes include hypertrophy of the smooth muscle cells, loosening of the intercellular matrix because of an increase in acid mucopolysaccharides, and decreased collagen production in the media.18–20 In addition to these structural changes, elevated cardiac output, increased total blood volume, and shearing forces during labor may cause increased arterial wall stress. This results in a separation of the layers of the arterial wall and creates a false lumen. The separation may be between the intima and the media, or between the media and the adventitia. Blood flow into the false lumen results in its expansion and can cause impingement of the true lumen of the coronary artery, impairing normal blood flow and causing myocardial ischemia, unstable angina, infarction, or sudden death (Figure 6-1). Thus, both hormonal and hemodynamic changes in pregnancy are thought to predispose women to intimal tears in the arterial wall and coronary artery dissections.
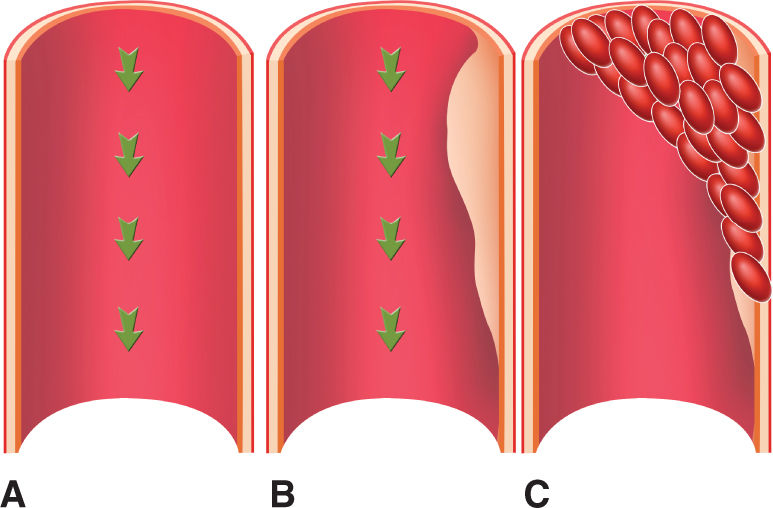
FIGURE 6-1. Coronary dissection. Normal tunica intima, media and adventitia, and blood flow (green arrows). (A) In a coronary dissection, there may be a separation between the intima and the media (B), or between the media and the adventitia. Blood flow into the false lumen results in its expansion and can cause impingement of the true lumen of the coronary artery, impairing blood flow and causing myocardial ischemia, unstable angina, infarction, or sudden death (C).
The profound alterations in the coagulation and fibrinolytic system that occur during pregnancy increase the risk for coronary thrombosis. Total 29% of patients in whom coronary artery anatomy was defined were found to have normal coronary arteries. Because a thrombus was found without atherosclerotic disease in 21% of the patients, a transient coronary spasm resulting in acute coronary thrombosis as a result of the hypercoagulable state of pregnancy was a possible explanation.21,22 Additional potential contributors to thrombus formation are the release of increased fast-acting tissue plasminogen activator (t-PA) inhibitor during placental separation, postpartum infection, blood transfusions, and thrombophilia (antiphospholipid syndrome, antithrombin III deficiency, protein C and S deficiency, and homocysteinemia). Because acute MI has been related to pregnancy-induced hypertension and preeclampsia, enhanced vascular reactivity to angiotensin II and norepinephrine and endothelial dysfunction may also promote coronary constriction.23–25 Other suggested causes of coronary spasm are decreased uterine perfusion in the supine position leading to renin release and angiotensin production and ergot derivatives that are used to control postpartum or postabortion hemorrhage or to suppress lactation.23,26–28 Coronary arterial spasm related to renin release from the transiently ischemic chorion is a proposed cause during gestation.29
Mortality
Although the maternal mortality rate previously ranged from 37% to 50%, improvements in diagnosis and treatment strategies have reduced the mortality rate to 5.1% to 11%.5–7 This improvement in mortality has been largely increased because of the use of percutaneous coronary intervention (PCI) in acute coronary syndromes (ACSs) in pregnancy. The mortality rate is higher in the peripartum period (18%) than in the antepartum and postpartum periods (both 9%). The incidence of fetal mortality was 9% (6 of 68), and most fetal deaths were associated with maternal mortality.5
DIAGNOSIS
As the technology for identifying and managing patients with MI has improved, the definition of MI has also evolved. In the past, a general consensus existed for the clinical syndrome designated as MI. In studies of disease prevalence, the World Health Organization in 1971 defined MI from symptoms and electrocardiogram (ECG) abnormalities. At the time, biomarkers for cardiac necrosis lacked specificity and reproducibility and therefore were not included in the definition. The highly specific and sensitive cardiac troponin (cTn) biomarkers of myocyte necrosis were subsequently introduced in the late 1980s, early 1990s, and became the basis of the first universal definition of MI in 2000 and its update in 2007. In 2000, the First Global MI Task Force presented a new definition of MI, which classified any degree of myocardial necrosis in the setting of myocardial ischemia as MI.30 In 2007, the Second Global MI Task Force further refined the definition of MI and included a five-category classification scheme.31 Significant developments of ever more sensitive and myocardial tissue-specific assays and more sensitive imaging techniques now allow for the detection of very small amounts of myocardial injury or necrosis leading to earlier diagnosis. In addition, the management of patients with MI has significantly improved, resulting in less myocardial injury and necrosis, in spite of a similar clinical presentation. This has now led to the Third Universal Definition of MI, which focuses on the detection of a rise and/or fall of cardiac biomarkers, with at least one of the values being elevated >99th percentile upper reference limit. The highly sensitive and specific cTn is the preferred biomarker of myocardial necrosis. In addition, one of the five following predefined criteria should be satisfied before a diagnosis of MI is made: (1) symptoms of myocardial ischemia; (2) new significant ST-segment/T-wave changes or left bundle branch block; (3) development of pathological Q waves on ECG; (4) new loss of viable myocardium or regional wall motion abnormality by imaging; and (5) identification of intracoronary thrombus by angiography or autopsy.32
The Third Global MI Task Force maintains that the ECG is an integral part of the diagnostic work-up in patients with suspected MI and should be obtained and interpreted in a timely manner.32 It also advocates the use of serial recordings to detect dynamic ECG changes. The task force outlined specific ECG criteria pertaining to the ST-segment shift and Q waves/QS complexes for the diagnosis of acute myocardial injury/ischemia and prior MI. In addition, the Third Global MI Task Force summarizes the ECG abnormalities that mimic myocardial ischemia or MI.32
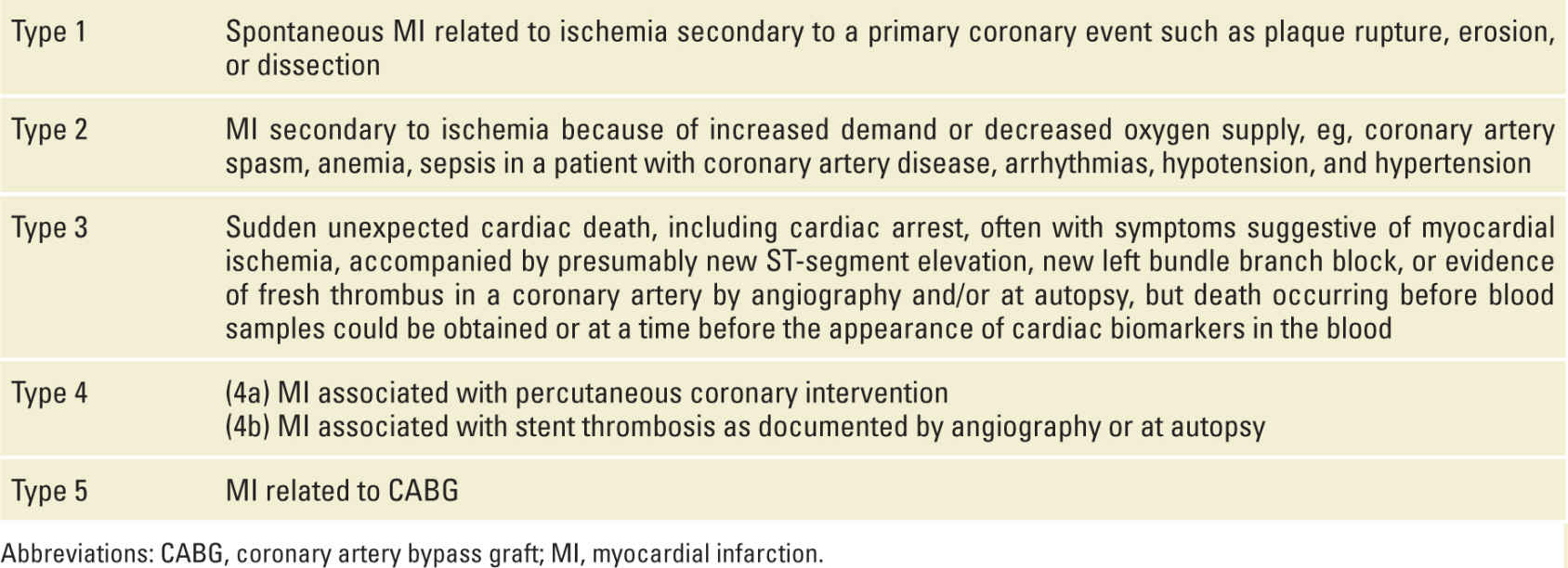
The Third Global MI Task Force updated the universal classification of MI into the following types: Type 1 MI is spontaneous MI induced by plaque disruption with overlying coronary thrombosis; Type 2 MI is induced by an imbalance in myocardial oxygen demand and supply, and an MI resulting in cardiac death is classified as Type 3, while Types 4 and 5 are PCI- and coronary artery bypass graft (CABG)-related MI, respectively32 (Table 6-1).
TABLE 6-1 | Classification Scheme for Acute MI |
cTns are a component of the myocardial contractile unit and serve as the cardiac necrosis biomarkers of choice for diagnosing MI. Myocardial necrosis results in myocyte membrane damage and the release of proteins into the circulation. In the event of an MI, cardiac-specific isoforms of troponin I and troponin T can subsequently be measured with great accuracy using commercially available assays that employ monoclonal antibodies specific to epitopes of these isoforms. These assays provide superior discrimination of myocardial injury when creatine kinase MB (CK-MB) levels are normal or minimally increased; they also impart additional prognostic information in patients who have elevated troponin levels despite normal CK-MB levels. In these patients, elevated cTn levels are associated with a higher risk of recurrent cardiac events. In addition, measurement of CK-MB can be impaired in the setting of major injury to other organs in which CK-MB is present such as the intestine, diaphragm, or uterus. A cTn level >99th percentile of the URL is considered elevated and is the cutoff level for a diagnosis of MI. This threshold value is determined for each specific assay in each laboratory and should be characterized by optimal precision, described by a coefficient of variation ≤10%. Blood samples for measuring cTn levels should be drawn serially on initial assessment and 3 to 6 hours later, when further ischemic episodes occur, or when the timing of the initial symptoms is unclear. To establish the diagnosis of MI, a rise and/or fall in values with at least one value above the decision level is required, coupled with a strong clinical suspicion.32
The Third Global MI Task Force reduced the emphasis on the use of other cardiac biomarkers. The use of cTn measurement is preferred over CK-MB, and the latter is to be used only when cTn assays are not available. The use of older nonspecific biomarkers including total CK, CK-MB activity, lactate dehydrogenase, aspartate aminotransferase, etc was not mentioned in the current recommendations. These markers are largely considered to be historical and should no longer be used alone to diagnose MI.32
Diagnosing acute MI in pregnancy and the postpartum period poses a great challenge to the physician for many reasons. In the pregnant patient who is usually regarded as young and healthy, there is usually a low level of suspicion for MI, which can lead to the misinterpretation of its signs and symptoms. In addition, the pathophysiology of MI in pregnancy is different and unlike ACS in nonpregnant women where acute MI is almost exclusively because of coronary atherosclerosis. As aforementioned, there are several different etiologies for acute MI in pregnancy. However, the criteria for diagnosis of acute MI in pregnant women are, in general, the same as in nonpregnant patients and include a constellation of symptoms, electrocardiographic changes, and cardiac markers. At the same time, however, the diagnostic approach is also influenced by fetal safety and the normal changes of pregnancy.33 Maternal safety is always more important than fetal safety in the decision-making process.
The diagnosis of pregnancy-related MI begins with an assessment of the patient and her clinical features. Pregnant women may or may not present with a preceding history of consistent with ACS. The patient may describe pain in her left chest behind the breastbone, her neck, left arm, or in her back between the scapulae. She may experience shortness of breath, diaphoresis, nausea, vomiting, palpitations, and anxiety. Women, in general, experience fewer of these symptoms than men and often have a more “atypical” or sometimes “silent” presentation of MI. However, in the context of pregnancy, the clinician may overlook the symptoms of MI as they overlap with the constellation of symptoms associated with pregnancy. Therefore, in a pregnant patient presenting with vague symptoms, it is thus important to rapidly evaluate the patient to identify signs of immediate life-threatening instability, and then to ensure that the patient is moved rapidly to the most appropriate environment for the level of care needed based on diagnostic criteria and an estimation of the underlying risk of specific negative outcomes.
As part of the initial assessment of the patient, an ECG should be performed if there is suspicion of cardiac involvement or acute MI. A 12-lead ECG is the single most important test in the initial evaluation of patients with suspected ACS and should be performed and reviewed within 10 minutes of the patient’s arrival.34 The presence of ST-segment elevation in two or more contiguous leads or a new left bundle branch block in the appropriate clinical scenario identifies patients who would benefit from emergent reperfusion therapy and the decision as to whether the patient will be treated with fibrinolytic therapy or primary PCI should be made within the next 10 minutes. Findings such as transient ST-segment elevation, ST-segment depression, and/or T-wave inversions support a high likelihood of ACS. Such patients need to be started on aggressive medical therapy, receive serial ECGs at 15- to 30-minute intervals, and need to be evaluated for early coronary angiography. Although a normal ECG reduces the likelihood of ACS, it should be remembered that the posterior and lateral walls are not adequately represented on the ECG and therefore may not completely exclude ischemia in those territories.35
Accompanying the ECG should be an evaluation of cardiac biomarkers. However, the interpretation of cardiac biomarkers may be complicating the changes that occur during normal labor and delivery. Shivvers et al. demonstrated that there is an increase in the concentration of CK and its MB fraction by nearly twofold within 30 minutes of delivery.36 This is likely caused by trauma during delivery to skeletal muscle, the uterus, and placenta (all of which contain a substantial amount of these enzymes). Mean CK-MB levels continued to rise and reached a maximum at 24 hours after delivery. In contrast, cTn levels are not similarly affected and demonstrate only minor increases after delivery that is below the upper limit of normal.36 Interestingly, studies in women with hypertensive disorders and preeclampsia in pregnancy have demonstrated elevated levels of cTn, which may indicate some degree of cardiac myocyte damage in these disorders.37,38 Despite this finding, cTns are the biomarkers of choice in the diagnosis of pregnancy-associated MI. An elevation in cTn >99th percentile of the normal range for the specified assay constitutes an abnormal test. Because it may take up to 6 hours following onset of myocardial necrosis for troponin to become elevated, an initial negative test should prompt a repeat test 6 to 9 hours later. Importantly, reperfusion therapy should not be delayed in patients with suspected ST-segment elevated MI (STEMI) until troponin elevation is documented. In patients without ST-segment elevation but suspected ACS, two negative tests 6 to 9 hours apart usually exclude non-STEMI.
The performance of an echocardiogram in the diagnosis of a pregnancy-related acute MI might be done safely to confirm the presence of ischemia by showing wall motion abnormalities that correspond to electrocardiographic changes. Echocardiography can also help with the exclusion of myocarditis, peripartum cardiomyopathy, and takotsubo-like syndromes, which could present with similar symptomatology. Although such an echocardiogram cannot be used to definitively rule out acute aortic dissection, it may detect an intimal flap in the proximal aorta or aortic valvular insufficiency. However, this study should only be performed if it is immediately available because “time is muscle” and additional studies should not markedly delay reperfusion therapy.
Exercise testing can be performed during pregnancy to aid in the diagnosis of myocardial ischemia or risk stratification following acute MI. Fetal bradycardia, decreased fetal heart rate variability, and the absence of body movement have been described during moderate to heavy maternal exercise.39,40 Therefore, the use of a submaximal protocol (70% of maximal predicted heart rate) with fetal monitoring, if possible, is preferred.41,42 The use of stress echocardiography may increase the sensitivity of the test for detection of myocardial ischemia and viability.41
If the index of suspicion of a cardiac ischemic event is high, despite any perceived lack of cardiac risk factors, urgent coronary angiography should be considered.13,16 There are numerous cases of angiography during pregnancy documenting generally favorable outcomes. Although the application of radiographic imaging during pregnancy is always of concern, the estimated radiation exposure to the fetus during coronary angiography is estimated to be only about 0.074 mGy.43 This level is well below the teratogenic threshold for any gestational age and is due in a large part from the use of a radial artery approach. Obtaining vascular access via the upper extremity has a logistic advantage over femoral artery access in the context of a pregnant patient, as it avoids direct fetal irradiation during catheter passage. The concern over more radiation exposure because of more fluoroscopy time required to perform coronary intervention using radial approach is theoretical, operator dependent, and has been recently challenged by Kuipers et al.44 Radiation can be further reduced by the use of appropriate abdominal shields.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


