Multifetal Pregnancy Reduction
As a byproduct of successful assisted reproductive technology, substantial numbers of women have conceived three or more fetuses over the past several years. These pregnancies are at increased risk for a variety of problems, but preterm delivery poses the greatest threat to the fetuses. The chance of delivering prematurely is directly proportional to the number of fetuses present, that is, as a group, triplets deliver earlier than twins, quadruplets earlier than triplets, and so on. This suggests that the preterm labor in these patients is a consequence of factors that might be favorably affected by simply reducing the number of fetuses present. The procedure referred to as multifetal pregnancy reduction (MPR) was designed to achieve that objective. This chapter presents the rationale for performing this procedure, describes the technical aspects of how it is accomplished, reviews its success, and briefly discusses some attending ethical issues.
Before proceeding, however, a brief discussion of nomenclature is appropriate. The foundation for fetal reduction was the elective termination of an anomalous twin. This type of procedure, whether performed in a twin pregnancy or one with more fetuses, is known as a selective termination. The term is appropriate because when one fetus in a multifetal pregnancy is known to be abnormal it is “selected” for termination, and that selection is the reason for performing the procedure. In the original series of fetal reductions performed in the first trimester, the authors referred to the procedure as “selective abortion,” which meant that some apparently normal fetuses were chosen to be terminated while others were left undisturbed (Dumez and Oury, 1986). In such procedures, the determining factor in the selection process is physical accessibility as opposed to fetal anomalies. Selection in this context is clearly very different from selection for an abnormal twin. Unfortunately, some authors failed to recognize this distinction, and early descriptions of the first trimester procedures refer to them as “selective reductions.” Recognizing the potential for misunderstanding among patients and the general public, Berkowitz and Lynch suggested in an editorial that the first trimester procedures described in this chapter be differentiated from selective terminations by referring to them as multifetal pregnancy reductions (Berkowitz and Lynch, 1990). The term multifetal pregnancy reduction says what it means and does not imply anything else. Purview of the recent literature indicates that this concept has been accepted, and the term nonselective embryo reduction has been adopted in the most recent American College of Obstetricians and Gynecologists (ACOG) Committee Opinion on the subject (Committee on Ethics, ACOG Committee Opinion, 1999).
NATURAL HISTORY OF MULTIFETAL GESTATIONS
Multifetal births account for a disproportionate percentage of the infant mortality rate, estimated at 9.7 deaths per 1000 live births for singletons, 52.7 deaths per 1000 live births for twins, and 138.5 deaths per 1000 live births for triplets (Jewell and Yip, 1995). The high mortality rates are closely related to the high rates of prematurity associated with multiple gestations. The incidence of pregnancy loss, maternal complications, and perinatal morbidity is also increased.
Four series published between 1988 and 1990 chronicle the natural history of 332 sets of triplets (Australian In Vitro Fertilization Collaborative Group, 1988; Lipitz, 1989; Newman, 1989; Gonen, 1990; and their colleagues). In these reports, delivery occurred at less than 37 weeks in 86–100 percent of cases, at less than 32 weeks in 20–39 percent of cases, and between 24 and 28 weeks in 3–10 percent of cases. The last interval is of greatest concern, of course, because of the increased possibility that surviving fetuses born at that time may suffer permanent damage as a consequence of their severe prematurity. The mean age of delivery for all infants in these series was 33 weeks, which is precisely the same as that of 110 sets of triplet neonates described in three earlier publications (with cases going back as far as 1946; Itzkowic, 1979; Holcberg, 1982; Syrop, 1985; and their colleagues).
Sassoon and colleagues compared 15 unreduced triplet and twin pregnancies that were matched for maternal age, race, type of medical insurance, delivery mode, parity, and history of preterm delivery (Sassoon and colleagues, 1990). These authors found that the women with triplets had significantly higher preterm delivery rates (87 vs. 26.7 percent) and longer neonatal hospital stays (29 vs. 8.5 days), with lower mean birthweights (1720 vs. 2475 g) and mean gestational ages at delivery (33 vs. 36.6 weeks). In a series of 165 triplets born in a single institution between 1992 and 1996, neonates born at 24 weeks or later had a 97 percent survival rate and a crude perinatal mortality rate of 121 per 1000 births (Kaufman and colleagues, 1998). Most triplets required neonatal intensive care unit (NICU) admission. Thirty-four percent required intubation, but only 3 percent developed chronic lung disease. The overall short-term neonatal outcome of triplets was similar to that of gestational-age-matched singletons and twins.
Essentially all women with quadruplet and quintuplet pregnancies deliver prematurely, with a perinatal mortality rate of approximately 25 percent in those patients reaching 24 weeks (Lipitz, 1990; Petrikovsky, 1989; and their colleagues). Although limited data are available for quadruplets, four series (Gonen, 1990; Lipitz, 1990; Vervliet, 1989; Collins, 1990; and their colleagues) reporting the outcome of a total of 89 sets of quadruplets, indicate that 98 percent delivered earlier than 37 weeks, 57 percent at less than 32 weeks, and 22 percent between 24 and 28 weeks.
Quintuplet outcome is limited to case reports, and only rare cases of surviving sextuplets have been reported. Two cases of surviving septuplets were highly publicized in 1997, followed by a report of a woman delivering octuplets in Texas. One octuplet did not survive the early neonatal period; there are no cases of surviving octuplets. Another case report details five surviving neonates from an octuplet pregnancy delivered at 33 weeks and reportedly normal at 2 years of age (Petrikovsky and colleagues, 1989).
Despite the recent publicized cases, pregnancy loss and extremely premature delivery are common with higher-order multiple gestations. Virtually nothing but case reports describe the outcome of pregnancies with five or more fetuses, but the perinatal outcome in those cases is considerably worse than for quadruplets. While controversy exists about the benefits of offering MPR to patients with triplets, there is very little doubt that the risks of delivering extremely premature infants for patients carrying four or more fetuses are excessively high.
TECHNICAL ASPECTS
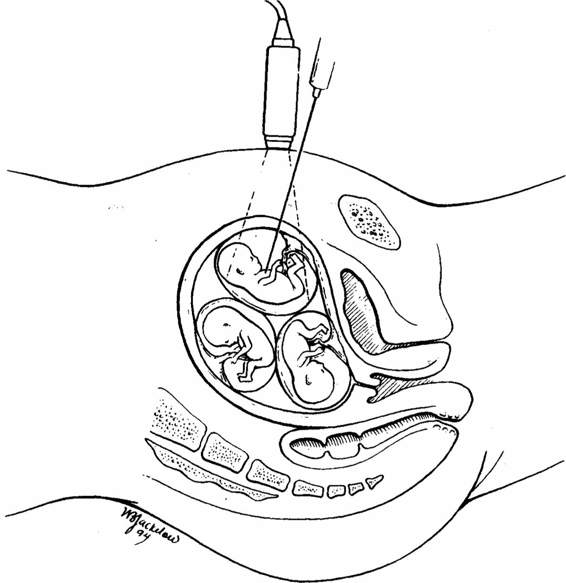
In 1986, Dumez and Oury published a series of 15 multifetal pregnancies reduced to four singletons, 10 sets of twins, and one set of triplets. Their technique was transcervical aspiration of one or more sacs under ultrasound guidance. At the time the report was written, nine women had delivered successfully, four pregnancies were ongoing, and two patients had lost their entire pregnancy. Anomalous twins had been selectively terminated prior to that time by a variety of techniques (Alberg, 1978; Kerenyi, 1981; Wittman, 1986; Beck, 1980; Rodeck, 1982; Antsakllis, 1984; Philip, 1985; and their colleagues), but this was the first description of procedures performed on presumably normal fetuses in order to reduce the potential for preterm delivery. In 1988, Berkowitz and associates published the first series of transabdominal MPRs. In this collection of 12 patients, the first three procedures were performed by transcervical aspiration, but the third patient experienced intractable bleeding during the procedure and it was necessary to terminate her entire pregnancy at that time. As a result of that experience, a technique was developed whereby a small quantity of potassium chloride was injected into the fetal thorax through a needle introduced transabdominally under ultrasonic guidance (Fig. 31-1). The following year Itskovitz and coworkers (1989) described a modification of this technique in which the needle was introduced transvaginally.
FIGURE 31-1. A needle is introduced transabdominally under ultrasound guidance into the fetal thorax and a small quantity of potassium chloride is injected.
Several relatively large series were published in the early 1990s describing the pregnancy outcome of patients undergoing multifetal pregnancy reduction (Lynch, 1990; Wapner, 1990; Boulot, 1990; Timor-Tritsch, 1993; and their colleagues). It would appear that most transcervical method investigators have abandoned the transcervical technique.
TRANSABDOMINAL APPROACH
Each woman referred for MPR undergoes an initial ultrasound examination to verify the number and crown-rump lengths of all viable fetuses. The couple is then counseled extensively about the risks and possible benefits of MPR. This session occurs at least 1 day prior to the. procedure in order to give the couple ample time to consider the pertinent issues and to have all questions answered. The procedure is performed in an outpatient setting (ultrasonography room). A single dose of antibiotic is administered prior to the procedure. An ultrasound examination locates the position of each fetus, confirms viability and crown-rump lengths, looks for morphologic abnormalities, and evaluates the thickness of the membranes separating all of the sacs. The operator and assistant then scrub and gown. The ultrasound transducer is placed in a sterile sheath. The maternal abdomen is prepped, draped, and covered with sterile scanning gel over the anticipated needle entry sites(s). A 22-gauge 9-cm needle is used for each insertion, unless longer needles are necessary. To minimize the risk of infection, no needle is ever reinserted into the maternal abdomen after it has been removed. Whenever possible, the fetus in the lowest sac is left alone unless it has an obvious anomaly, has an abnormally small crown-rump length, or appears to be a member of a monochorionic set of twins. A needle is then introduced under direct ultrasonic guidance into the selected fetus, and 2–3 mEq of potassium chloride is injected (Fig. 31-1). If cardiac activity persists, additional potassium chloride is administered. The needle is left in place for 3 minutes after asystole is confirmed in case there should there be resumption of cardiac activity. The same procedure is repeated for other fetuses undergoing termination. When six or more fetuses are present, the reductions are usually done in two or three sessions separated by 1-week intervals. After completion of each procedure, the patient is rescanned to document presence or absence of cardiac activity for each fetus. An hour later, a final scan is performed to rule out the small possibility that fetal cardiac activity had resumed in a “reduced” fetus. The woman is advised to limit her activities for the following 48 hours and to report any episodes of significant bleeding, loss of fluid, contractions, or signs of early infection. Follow-up ultrasound examination 2 weeks later is recommended.
In a series of 200 completed cases from New York’s Mount Sinai Hospital, 89.5 percent were done between 11 weeks and the end of the twelfth week (Berkowitz and colleagues, 1993). The other 21 cases were performed outside this range but later than 9 weeks 4 days and prior to 13 weeks 3 days. Several reasons were cited for choosing this narrow interval. First, although it is technically feasible to do the procedure earlier, it is more difficult to accomplish with a smaller fetus. Second, by waiting until 11 weeks, there is a greater opportunity to see a morphologic abnormality or evidence of diminished growth. Third, there is a greater potential for spontaneous death in utero of one or more fetuses than when the procedure is done earlier. Finally, if one waits more than 12 completed weeks, the advantages of further delay must be weighed against the increased residual fetal mass.
In the same series, the average duration of the procedure was 6.6 minutes per fetus, with a range of 2–30 minutes (including 3 minutes of observing asystole with the needle still in place). In 93 percent of the cases, the amount of potassium chloride used was 5 mEq per fetus or less, with a range of 2–10mEq.
TRANSVAGINAL APPROACH
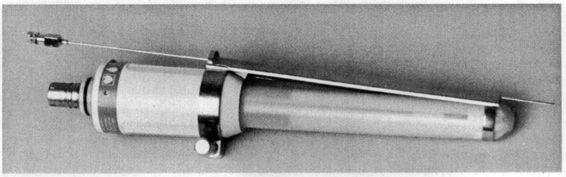
The transvaginal approach uses a needle guide or automated spring-loaded device that attaches to a transvaginal ultrasound transducer (Fig. 31-2). With this approach, the procedure can be performed considerably earlier in gestation; however, the chance of detecting a morphologically abnormal fetus is reduced. As a consequence, in the largest study of transvaginal procedures, Timor-Tritsch and associates (1993) chose to perform the procedure after 9.5 weeks. There is another theoretic concern: although the sac over the internal os is the easiest to reach with the transvaginal approach, death of the fetus in that sac could predispose the patient to ruptured membranes later in the pregnancy. This latter complication has not been proven to be an important clinical problem (Timor-Tritsch and colleagues, 1993). Finally, the risk of introducing infection may be greater when the vaginal route is used. In the aforementioned study, there were three clinically apparent infections following 134 procedures. Two of those infections responded to antibiotic therapy, but the third pregnancy required termination. The vaginal approach is also easier in obese women and in those with substantial abdominal scarring.
FIGURE 31-2. A needle guide or automated spring-loaded device attaches to a transvaginal ultrasound transducer.
Similar to the transabdominal approach, it is recommended that a vaginal prep be performed with Betadine soap and paint solutions immediately prior to the procedure. Use of a broad-spectrum antibiotic is also recommended. Centers using this approach also routinely obtain cervical cultures for chlamydia and Group B streptococcus prior to the procedure (Timor-Tritsch and colleagues, 1996).
GENETIC TESTING
Patients undergoing MPR may have coexisting indications for genetic testing, such as the maternal-age-related risk for chromosomal abnormalities. Patients at risk for autosomal recessive or dominant disorders face a similar dilemma when considering options regarding the prenatal diagnosis and MPR procedures. Although some major morphologic anomalies can be detected with ultrasound at the time of the MPR procedure, most karyotypic abnormalities will not be discovered unless chorionic villus sampling (CVS) or early amniocentesis is performed. The use of either of those invasive procedures, however, may increase the risks of pregnancy loss, as well as possibly delay the time at which the reduction is performed. In addition, two recent randomized studies question the safety of amniocentesis performed before 12 weeks of gestation (Canadian Early and Mid-trimester Amniocentesis Trial [CEMAT] Group, 1998; Sundberg and associates, 1997). Definitive identification of the source of each villus sample may be extremely difficult, especially if more than three fetuses are present.
Brambati and colleagues diagnosed five cases of aneuploidy, four cases of thalassemia major, and one case of congenital adrenal hyperplasia by CVS prior to MPR (Brambati and colleagues, 1995). Diagnostic errors occurred twice (1.5 percent) because of incorrect sampling. No significant difference was found in the rate of pregnancy loss between the group undergoing CVS and MPR and the group undergoing MPR (2/69 vs. 5/31). Similarly, a series of 25 patients undergoing CVS prior to MPR by De Catte and associates showed no added risk of the diagnostic procedure (De Catte and colleagues, 1998). The study was limited by a relatively small number of patients, but no fetal losses occurred and no deliveries occurred prior to 28 weeks’ gestation. In most cases, testing was performed on the embryos not chosen to be reduced. In the largest series to date, Eddleman and colleagues reported 166 CVS procedures performed in 86 pregnancies prior to MPR. CVS was successfully performed in 99.4% of cases, and only 1 of 73 patients beyond 24 weeks at the time of report had a pregnancy loss after MPR. The group estimated the sampling error at 1.2%, but noted it could potentially be higher (Eddleman and colleagues, 2000).
These results suggest that CVS prior to MPR is technically feasible and does not increase the risk of pregnancy loss after MPR. While these results are encouraging, questions remain regarding the sampling error associated with the technique in multiple gestations, and patients should be counseled of this possibility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree