Multifetal Gestations
In 1997, the 110,874 multifetal gestations born in the United States were the highest number ever recorded. The frequency of multiple birth has been steadily increasing since 1980. Over the past 20 years, there has been a 42 percent increase in the frequency of twin and a 469 percent increase in triplet and higher order births (Ventura and colleagues, 1999). The frequency of monozygotic twinning is virtually the same in all populations at 4 per 1000 births. The incidence of dizygotic twinning is independent of age, race, parity, and heredity. The incidence of dizygotic twinning, on the other hand, is extremely variable and accounts for most of the increase in multifetal births seen over the past two decades. Approximately one-fourth of the increase in dizygotic twinning has been attributed to delayed childbirth and the naturally higher occurrence of multifetal births in older mothers. The majority of the remaining increase is the result of fertility enhancing agents and assisted reproductive technologies where the risk of a multifetal birth may be as high as 25 percent (Jewell and colleagues, 1995). Other influences on the rate of dizygotic twinning include family history, racial background, and parity. For example, dizygotic twinning is more common in black Americans than in whites and even less common in those of Asian ancestry. With regard to heredity, it appears that the maternal genetic background is more important than the paternal contribution (Callahan and colleagues, 1994).
PERINATAL IMPLICATIONS
Although multifetal births account for only approximately 2 percent of all live births, they are responsible for a disproportionate share of all perinatal morbidity and mortality. Multifetal gestations account for 13 percent of preterm births (<37 weeks), 15 percent of early preterm births (<32 weeks), 21 percent of low-birthweight infants (<2500 g), and 25 percent of very-low-birthweight infants (<1500 g) (Ventura, 1999; Powers, 1994; Donovan, 1998; Stevenson, 1998; and their colleagues). The average birthweight and gestational age for singleton gestations is 3358 g at 39.3 weeks, as compared to 2500 g at 36.2 weeks for twins and 1698 g at 32.2 weeks for triplets (Martin and colleagues, 1997). As a result of these very high rates of both prematurity and low birthweight, twins are at an approximately sevenfold greater risk of dying before their first birthday than are singletons, while triplets are at almost a 20-fold greater risk (Luke, 1994; Kiely, 1992; Luke, 1992; Newman, 1998; and their colleagues). Among the survivors, multifetal gestations also have an increased risk of mental and physical handicaps (Luke and colleagues, 1992; Newman, 1998). Twin pregnancies produce a child with cerebral palsy 12 times more often than do singleton pregnancies, while one-fifth of triplet pregnancies and one-half of quadruplet pregnancies result in at least one child with a major long-term handicap (Grether, 1993; Yokoyama, 1995; and their colleagues). Importantly, the increased risk of cerebral palsy in multifetal gestations is not solely related to a greater risk of prematurity. When matched for gestational age, infants of multifetal gestations have a nearly threefold greater risk of cerebral palsy (Grether, 1993; Mutch, 1992; and their colleagues). Minakami and colleagues (1998) reviewed the frequency of death, cerebral palsy, and mental retardation in 136 twins conceived by artificial reproductive techniques as compared with 72 spontaneously conceived twins, and reported a lower frequency of adverse outcomes in the former versus the latter group (3.3 percent vs. 8.3 percent). Other factors associated with an increased risk of handicap among multifetal births are preeclampsia, preterm premature rupture of the membranes, death of a cotwin in utero, significant birth-weight discordancy, and mode of delivery (Mauldin and Newman, 1998).
There is a negative synergism between prematurity and low birthweight. Growth-retarded premature infants, regardless of plurality, have a significantly higher risk of morbidity and mortality than do appropriately grown infants of the same gestations age (Kilpatrick, 1996; Luke, 1993; Wolff, 1992; and their colleagues). Growth-retarded twins and triplets experience an excess of neurodevelopmental abnormalities compared to appropriately grown, gestational age-matched multiples (Low, 1992; McCormick, 1992; and their colleagues). Perinatal morbidity and mortality is also several-fold higher for monozygotic twins (Kovacs and colleagues, 1989). Congenital anomalies are approximately twice as common in twins as they are in singletons with most of this increase occurring among the monozygotic gestations (Edwards and colleagues, 1995). Monozygotic twins may also be monochorionic with vascular communications placing them at risk for twin-to-twin transfusion syndrome. Other factors associated with increased perinatal morbidity and mortality in multifetal gestations include monoamnionicity, prolapse of the umbilical cord, premature placental separation, intrapartum asphyxia, malpresentation, and birth trauma.
MATERNAL IMPLICATIONS
Not unexpectedly, multifetal gestations are also associated with significantly higher maternal morbidity and health care costs. Women pregnant with multifetal gestations are nearly six times more likely to be hospitalized during pregnancy with complications such as pregnancy-induced hypertension/preeclampsia, preterm labor, preterm premature rupture of the membranes, placental abruption, pyelonephritis, and postpartum hemorrhage (Gardner, 1995; Ellings, 1993; Spellacy, 1990; Haas, 1996; Albrecht, 1996; Peaceman, 1992; Newman, 1989a; and their colleagues). Luke and colleagues (1996) determined that hospital costs for the birth admission of women with multifetal gestations averaged almost 40 percent higher than for gestational age-matched singletons primarily because of longer length of stays both before and after delivery and their greater rate of obstetric complications.
A common and dangerous maternal complication associated with multifetal gestations is pregnancy-induced hypertension. Preeclampsia frequently occurs earlier, is more severe, and is more often atypical in multifetal gestations. In a review of 341 twin pregnancies, Thompson and colleagues (1987) reported an 18 percent incidence of pregnancy-induced hypertension. Long and Oates (1987) reported a 26 percent incidence of hypertension in 642 twin gestations. In the latter report, almost three-fourths of the cases occurred prior to 37 weeks gestation. Hardardottir and colleagues (1996) reported that the incidence of preeclampsia was 19 percent in triplet pregnancies, 38 percent in quadruplets, and higher if the patient was nulliparous. Hypertension is not always the presenting sign, nor is proteinuria universally present. Only 3 of 16 triplets and quadruplets reported by Hardardottir met the traditional criteria for preeclampsia. The most common presentation among these high-order multiples was maternal symptoms typical of severe preeclampsia associated with laboratory abnormalities consistent with the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome.
Other maternal complications encountered with increasing frequency in multifetal gestations include cholestatic jaundice, pruritic urticarial plaques and papules (PUPP) of pregnancy, urinary tract infections, and respiratory embarrassment. Women with multifetal pregnancies also experience an increasing number of various somatic complaints such as shortness of breath, loss of balance, varicose veins, pruritic stretch marks, significant dependent edema, constipation, and hemorrhoids.
GENERAL PRINCIPLES OF INTRAPARTUM MANAGEMENT
Successful intrapartum management of multifetal gestations requires careful preparation (Table 9-1) and attention to several important general principles. First and foremost is the assemblage of experienced and skilled personnel. The obstetric team should be familiar with the risks posed by multifetal gestations, and should include someone experienced with both operative and manipulative delivery techniques. The team should include another physician or nurse capable of monitoring the progress of labor, assisting with delivery, and performing intrapartum ultrasound. Other necessary personnel include an anesthetist and a neonatal team sufficient to resuscitate two or more infants.
TABLE 9-1. Preparatory Checklist for Delivery of a Multifetal Gestation
Skilled obstetric attendants (2) for labor and delivery
Anesthesiologist available at delivery
Neonatal care personnel sufficient for resuscitation of all newborns
Portable ultrasound scanner
Reliable intravenous access (16–18 gauge)
Cardiotocograph with dual monitoring capability
Delivery bed with lithotomy stirrups
Obstetric forceps (Piper’s if breech delivery planned) or vacuum apparatus
Pre mixed oxytocin infusion
Tocolytic agent of choice for uterine relaxation
Methergine and/or 15-methyl prostaglandin F2α
Immediate availability of blood
Capabilities and staff for an emergent cesarean section
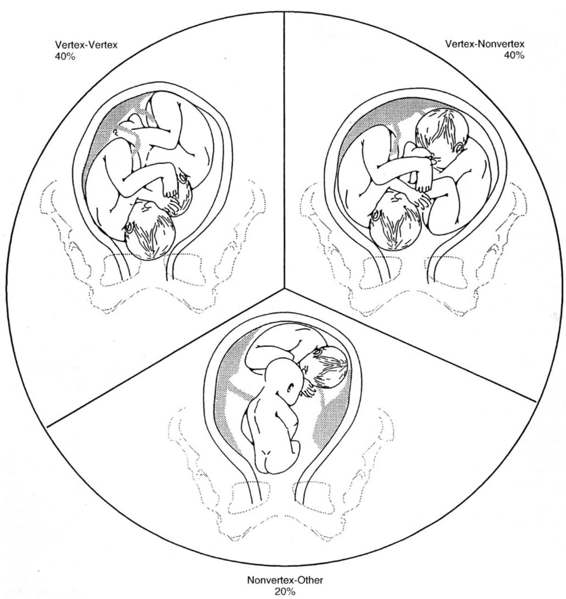
A second principle of intrapartum management for multiples is to ascertain presentation and estimate the fetal weight of each fetus on admission to the labor and delivery unit. The conceptual framework most commonly used to categorize the intrapartum management of twin gestations is based on their relative presentations (Fig. 9-1). These categories include those twins who both present as a vertex, when the first twin is vertex and the second is nonvertex, and when the first twin is in a nonvertex presentation. These categories are distributed as approximately two-fifths, two-fifths, and one-fifth respectively (Chervenak and colleagues, 1985). Repeating the ultrasound assessment after delivery of the first twin is also important, as the second twin may change position after delivery of the first in as many as 20 percent of cases (Trofatter, 1987).
FIGURE 9-1. Distribution of the three major categories of fetal presentation in twin gestations.
Successful intrapartum management of multifetal gestations also requires continuous and simultaneous monitoring of both fetuses in labor. This is most efficiently achieved by electronic fetal heart-rate monitoring. Multifetal gestations are at increased risk for several intrapartum complications that could manifest as an abnormal fetal heart-rate pattern. These include dysfunctional uterine contractility, premature separation of the placenta (especially after the birth of the first twin), prolapse of the umbilical cord, cord entanglement, or uteroplacental insufficiency, which is a greater risk when one of the twins has been identified as having intrauterine growth restriction or significant growth discordance. External monitoring of both fetuses is acceptable; however, care must be taken to ensure that the same twin is not being simultaneously monitored. Alternatively, when the membranes of the first twin have ruptured, that twin may be monitored directly with a fetal scalp electrode while the second is monitored externally.
Ready availability of anesthetic services is also critical for consistently successful intrapartum management. Because of the frequent and sometimes emergent need for operative or manipulative obstetric procedures, epidural analgesia is often the anesthetic approach of choice (Crawford, 1987). While continuous epidural analgesia has proven highly effective for both labor and delivery in multifetal gestations, it has also been reported to prolong the second stage of labor (Crawford, 1987). Less-attractive alternatives to epidural analgesia include intravenous narcotic analgesia for labor and pudendal block with perineal infiltration of local anesthetic for delivery. If intrauterine manipulation should become unexpectedly required, general anesthesia with a halogenated agent may be necessary to achieve uterine relaxation. Uterine relaxation may also be achieved with either nitroglycerin or terbutaline. Both conduction and general anesthesia are appropriate for the cesarean delivery of a multifetal gestation.
A final principle of intrapartum management is to ensure the availability of blood. Multifetal gestations experience an increased average blood loss with delivery and are at increased risk of postpartum hemorrhage due to uterine atony. Multiples are also at increased risk for placental abruption, placenta previa, and cesarean section. These risks are frequently superimposed on maternal anemia, which is one of the most common complications associated with multifetal gestations.
LABOR
Compared to singleton gestations, multifetal pregnancies experience an increased baseline uterine contraction frequency during the latter half of gestation (Alvarez, 1950; Newman, 1986; and their colleagues). This increased baseline uterine contractility leads to advanced prelabor cervical changes. Almost 40 percent of nulliparous and 60 percent of parous women with multifetal gestations present in labor with a cervix dilated >3 cm as compared to 7 percent and 23 percent of singleton pregnancies, respectively (Friedman and colleagues, 1964). As a result of this prelabor cervical change, the latent phase is typically shorter in multiple gestations than in singletons. However, the active phase of labor in multiples is typically lengthened compared to singleton norms. Prolongation of the active phase in multifetal gestations is more pronounced for nulliparous women than for parous women. This relative uterine inertia during active phase is usually ascribed to uterine overdistension and a greater frequency of malpresentation. Uterine overdistension results in an increased frequency of contractions that are of a lesser intensity (Alvarez and colleagues, 1950). This pattern of uterine activity is referred to as hypotonic uterine dysfunction and is not uncommon in multifetal gestations.
Oxytocin induction and/or augmentation of labor are both acceptable in multifetal gestations although oxytocin should be used judiciously as is always the case when there is uterine overdistension. The longer active phase in multiples counterbalances the shorter latent phase resulting in a similar overall duration of labor for single and multifetal gestations (Friedman and colleagues, 1964). Suzuki and colleagues (2000) published a clinical trial of induction of labor versus expectant management in twin pregnancies. The induction group received oral prostaglandin E2. Birthweights, cesarean delivery rates, and outcomes were similar in both groups.
Multiple factors influence the duration of the descent phase. Overall, it is slightly lengthened due to the presence of multiple fetuses. The typical smaller size of multifetal gestations should allow for a more rapid descent, although other factors, such as malpresentation and the possibility of collision, may offset this advantage.
There is often a period of hypocontractility following delivery of the first twin. The laxity of the uterine wall resulting from reduced intrauterine volume must be taken up by aggressive retraction of the uterine muscle before effective uterine contractility can resume. To shorten this lag phase, a previously prepared oxytocin infusion can be started.
The third stage of labor also deserves comment. Multi-fetal gestations are at increased risk of postpartum hemorrhage associated with uterine atony. In anticipation of a possible hemorrhage, oxytocin should be started immediately after delivery of the second twin if not already begun. Twenty to 40 units of oxytocin can be added to 1000 mL of intravenous crystalloid solution and infused at 100–200 cc an hour. If bleeding is excessive, methylergonovine 0.2 mg can be given intramuscularly or via a transabdominal intramyometrial injection. Chronic or pregnancy-induced hypertension is a not uncommon contraindication to the use of methylergonovine. Another effective alternative is the intramuscular or intramyometrial injection of .25 mg of 15-methyl prostaglandin F2α. 15-Methyl prostaglandin F2α can be repeated a second time in 10–15 minutes if the bleeding has not abated. Consideration should be given to continuing the oxytocin infusion for several hours after delivery to further reduce postpartum bleeding.
DELIVERY
Delivery of multifetal gestations requires the anticipation of unpredictable events and the need to consider at least three separate patients. It is a tremendous challenge for obstetric care providers to ensure the best possible outcome for mother and babies. Unfortunately, there is a conspicuous absence of strong clinical evidence to guide decision making. With the exception of the single randomized controlled trial of Rabinovici and colleagues (1987), virtually all the literature addressing the intrapartum management of multifetal gestations are either observational or nonrandomized comparative trials. While a tremendous amount of clinical experience is presented in these studies, they may ultimately create a false impression. Such studies suffer from numerous methodologic deficiencies, including the difficulties inherent in retrospective data collection, selection bias, and un-considered confounding variables. It is conceivable that differences in outcomes reflect clinical differences upon which the mode of delivery was chosen, rather than on the mode of delivery’s efficacy.
VERTEX/VERTEX
It is generally agreed that the 40 percent of twins who present with both in a vertex presentation should be delivered vaginally, and that a high likelihood of success is anticipated (Chervenak, 1984; Cetrulo, 1986; Laros, 1988; Chervenak, 1985; and their colleagues). Despite speculation, there is no evidence that vertex vaginal delivery of the very-low-birth-weight twin (< 1500 g) is associated with any increased risk of perinatal mortality or interventricular hemorrhage (Morales and colleagues, 1989).
After delivery of the first twin, the presenting part of the second twin should be confirmed by pelvic examination or by real-time ultrasonography. If labor has not resumed within 10 minutes of the delivery of the first twin, oxytocin should be initiated. As throughout labor, it is important that continuous heart-rate monitoring of the second twin is assured. The fetal head should be identified within the pelvic inlet with special attention to excluding either a compound or funic presentation. After effective uterine contractions are reestablished, the woman is encouraged to again bear down in order to achieve further descent. Amniotomy should be performed during a contraction and with moderate fundal pressure applied to help fix the vertex within the pelvis. The amniotic sac can be ruptured grossly if the fetal head is well applied to the cervix or leaked with a spinal needle if the vertex is not well applied.
Previous data suggested that the time interval between the twin deliveries should not exceed 30 minutes. This recommendation has been dramatically altered by the development of electronic fetal monitoring, intrapartum ultrasound, and intensive neonatal support. Much of the data suggesting higher perinatal morbidity and mortality associated with long delays between delivery of the two twins describes an era when the second twin was frequently not recognized until after delivery of the first (Farooqui and colleagues, 1973). In 1975, Jouppila and colleagues began to question the wisdom of an arbitrary time limit between the delivery of the first and second twin. Rayburn and coworkers (1984) reviewed 95 twin pairs in 1984, and reported that the mean time interval between delivery of twins was 21 minutes with a range of 1 minute to more than 2 hours. Delivery of the second twin occurred in 15 minutes or less in almost two-thirds of the cases. However, when the time interval was >15 minutes, there was no excess trauma or fetal compromise. Rayburn concluded that expectancy between the delivery of the two twins was associated with the lowest risk of perinatal morbidity. Delays of more than 1 hour have not been associated with adverse outcomes for the second twin as long as continuous fetal heart-rate monitoring is used (Feng and colleagues 1995).
In some cases, deterioration of the fetal condition may occur before a safe vaginal delivery is possible. Some complications such as premature placental separation or prolapse of the umbilical cord are clinically apparent; in other cases, deterioration of the fetal heart rate tracing may be indecipherable. Fetal distress of the second twin should usually be managed by immediate cesarean delivery or by operative vaginal delivery. High vacuum or forceps deliveries should be avoided except in the most emergent of situations, and even then, it should be considered only when an experienced operator is present and while simultaneously preparing for an emergent cesarean. Similarly, internal podalic version should only be considered when emergent delivery is mandated and cesarean delivery is not an immediately available option. There are no current series documenting the safety of internal podalic version in cases of fetal distress. Because of the possibility of intrapartum fetal distress and the limited options for effectively dealing with that situation, access to immediate cesarean delivery should be available if possible.
INTERNAL PODALIC VERSION
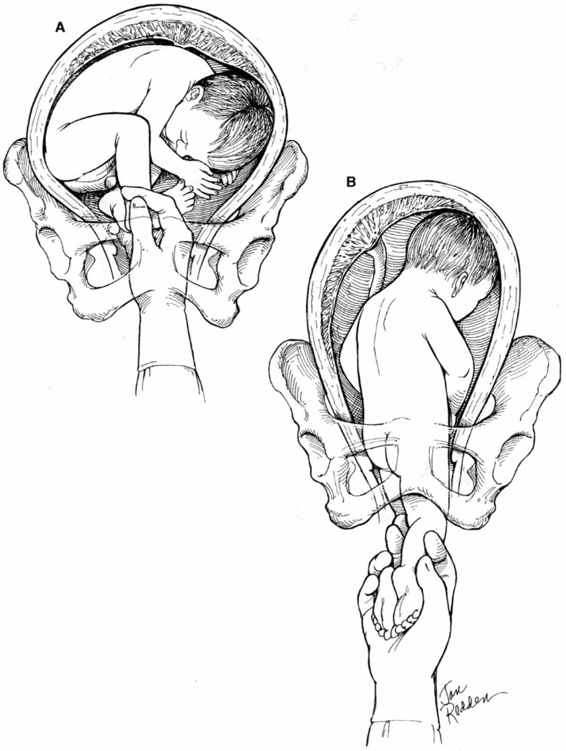
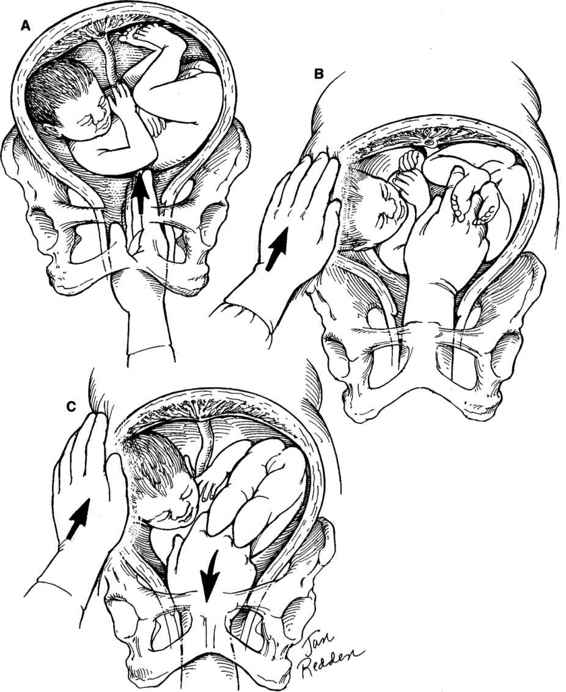
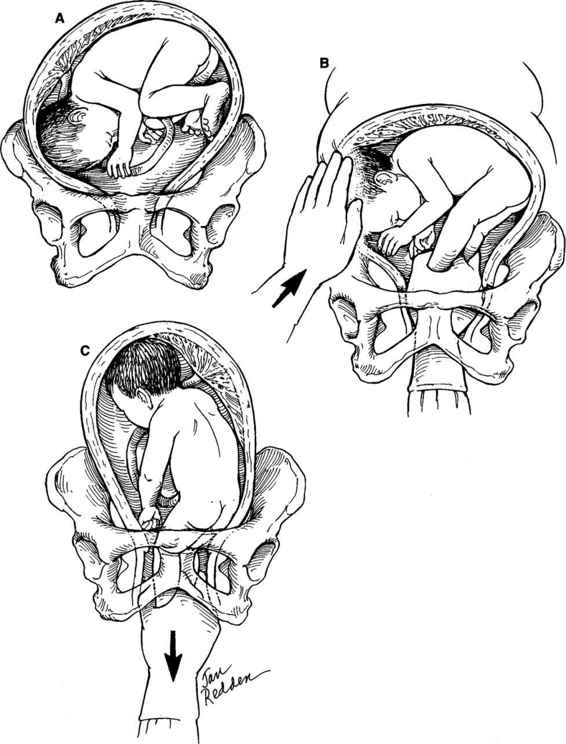
Internal podalic version is now an infrequently performed procedure. The procedure varies somewhat depending on whether the fetus is in a ventral (Fig. 9-2), scapular (Fig. 9-3), or vertex presentation (Fig. 9-4). The procedure is performed most easily when the fetus is in the ventral position. A greater degree of skill is required when the internal version and breech extraction are performed after a vertex presentation has failed to descend into the pelvis. The obstetrician usually places one hand externally on the uterine fundus with the other placed transvaginally into the uterus. If the fetal head is obstructing the lower uterine segment or pelvic inlet, it should be displaced with the vaginal hand to the point that the abdominal hand can sweep it up into the upper uterus. The next step is to then identify and grasp both feet of the fetus. Both feet should be brought down into the vagina prior to rupture of the membranes if possible; if not, a single foot can be delivered first and then the second located as the fetus descends through the birth canal. After the feet have been successfully brought down into the birth canal, the membranes can be ruptured and a breech extraction carried out. Often the membranes have ruptured prior to grasping the fetal feet, or it may be necessary to rupture the membranes in order to correctly identify the fetal feet. Care must be taken to avoid simultaneously grasping one foot and one arm.
FIGURE 9-2. Internal podalic version: ventral presentation. A. The ventral surface of the infant is directed toward the pelvic inlet with the feet in easy position to be grasped with little intrauterine manipulation. The obstetrician’s hand and forearm are introduced through the vagina and cervix into the uterus, and the exact position and attitude of the infant determined. The feet are grasped and pulled through the cervix. B. After the feet have been brought out through the introitus, the membranes are ruptured if they have not previously done so. The buttocks will follow, and the head will automatically assume a position in the fundus of the uterus. Although it is not necessary to apply external pressure in an attempt to push the head upward, this is a reasonable practice to institute with every internal version. Extraction is then performed as for a primary breech position.
FIGURE 9-3. Internal podalic version: scapular presentation. A. The presenting shoulder is pushed upward well out of the pelvis. B. The vaginal hand and the abdominal hand simultaneously assist with elevating the presenting part. Either hand may be used as the vaginal hand and will depend on operator preference and position of the fetus. C. Both feet are identified and grasped with the vaginal hand. In the left scapular anterior position, the feet are shown being pulled anteriorly and downward. Simultaneously, the baby’s head and shoulder are lifted out of the pelvis. The delivery is completed by sweeping the head and shoulders into the fundus with the abdominal hand as the feet are drawn down into the lower birth canal with the vaginal hand. This procedure is greatly facilitated by uterine relaxation and intact membranes.
FIGURE 9-4. Internal podalic version: vertex presentation. A. For a baby in the vertex position, the abdominal and vaginal hands are used simultaneously and in coordination. The head often is at the level of or slightly lower than the feet. The vaginal hand is used to elevate the head to the abdominal hand, which then sweeps it up into the fundus. B. Simultaneously, the vaginal hand locates the feet. C. After the feet have been grasped, they are brought down into the lower birth canal, the membranes ruptured, and the remainder of the breech extraction performed.
An assistant capable of providing simultaneous real-time ultrasound may be helpful in identifying the exact position of the fetus throughout the procedure. The anesthesiologist can be of assistance by providing 50–100 μg of nitroglycerine intravenously for uterine relaxation. Alternatively, uterine relaxation can be achieved with 0.25–0.5 mg of terbutaline sulfate administered subcutaneously.
Internal podalic version is probably most difficult when the infant is in a left or right scapular anterior position. The obstetrician’s hand must be introduced deeper into the uterus in order to reach the feet, and more manipulation is required to rotate the back upward and to simultaneously correct the transverse lie. In addition, the arm may be prolapsed, which further complicates the procedure. Uterine relaxation will likely be required to effect this delivery. In the absence of a highly experienced operator and excellent anesthesia, prompt delivery by cesarean may be a more prudent choice.
After delivery, the placentae can be delivered by manual removal if the uterus is still adequately relaxed and analgesia is sufficient. The uterus should be carefully explored for defects or retained products. After the uterine exploration has been completed, the uterine-relaxing agent is discontinued and oxytocin initiated to help achieve myometrial contraction.
The clinical experience with internal podalic version and subsequent breech extraction is limited in most training programs. In a review of 578 sets of twins delivered between 1980 and 1987 in Halifax, Nova Scotia, Adam and colleagues (1991) reported 18 uneventful internal podalic versions and breech extractions in second twins weighing ≥ 1500 g. Internal podalic version and breech extraction was also performed in six second twins weighing 1000–1499 g, of whom two experienced an intraventricular hemorrhage. In one of the two cases, both twins had severe respiratory distress syndrome (RDS) and grade III intraventricular hemorrhages, making it difficult to implicate the version and delivery. However, in the second case, the authors acknowledged that a difficult delivery was the likely cause for a low Apgar score and intraventricular hemorrhage in twin B only.
Internal podalic version and breech extraction of the second twin may be considered when (a) there is a clear obstetric indication; (b) the fetus has an estimated fetal weight ≥1500 g; (c) the operator is experienced in the performance of internal podalic version and breech extraction; (d) anesthesia is available for effective uterine relaxation; and (e) emergent cesarean delivery can be performed if necessary. Because there are little, if any, meaningful data regarding internal podalic version and breech extraction of the second twin with an estimated weight of less than 1500 g, experience and judgment are of paramount importance in choosing the most appropriate mode of delivery. Many clinicians prefer cesarean delivery for this latter situation.
VERTEX/NONVERTEX TWINS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree