16 MRI-Guided Interventions Equipment and procedures used for MRI-guided interventions must accommodate the special conditions encountered during an MRI scan. This applies especially to the extremely strong magnetic field, and to the short time slot of only a few minutes during which the hypervascularized target is visible. The materials used (needles, wires, clips, and coils) must therefore have favorable ferromagnetic characteristics so that they are easily identified within the breast but do not cause susceptibility artifacts that will interfere with image acquisition and target identification. In addition, measurement sequences that show as little vulnerability as possible to signal extinction due to susceptibility artifacts must be chosen for MRI-guided interventions. First attempts at MRI-guided preoperative localization were made in the 1990s using the so-called “free-hand” technique. For this, cutaneous markers were placed on the skin for better orientation and the position of the lesion was estimated. Because this method is relatively inexact, it was quickly abandoned. Later, dedicated puncture devices for interventions in the supine, oblique, or sitting body positions, depending on the particular work group, were developed using appropriate breast surface coils. These systems were used primarily for MRI-guided preoperative localizations, and increasingly also for large-core and vacuum-assisted biopsies, mostly with a lateral approach. The decisive breakthrough for the routine and reliable performance of percutaneous MRI-guided biopsies occurred with the development of open breast surface coils into which dedicated targeting devices can be integrated. Nowadays MRI-guided breast interventions are performed using open surface coils that allow access to the breast and integration of a dedicated puncture device. Open surface coils that also allow the integration of compression paddles for diagnostic examinations are available from various companies (Fig. 16.1). Two systems are usually used to calculate and mark the appropriate puncture coordinates, as well as to guide the puncture needle: Several different companies offer software for the calculation of the appropriate puncture coordinates in the x-, y-, and z-axes for MRI-guided breast interventions. In principle, the coordinate data are calculated in relation to an arbitrarily chosen zero-point, from which the distance to the target lesion is calculated in the three spatial planes. Currently available systems include SureLoc from Confirma Europe Corp., DynaLOC from Invivo Corp., and MICS MIA from Machnet B.V. Fig. 16.2 Post-and-pillar targeting equipment for MRI-guided breast interventions. NORAS Co. breast biopsy unit with compression grid. Continuous adjustment of the x– and y-axes is possible by sliding the telescopic post-and-pillar system. Compatible with all customary open surface coils. Fig. 16.3 Biopsy and localization grid for MRI-guided breast interventions. NORAS Co. breast biopsy unit with compression grid with chambers for the insertion of perforated guide blocks with various puncture opening sizes. Compatible with all customary open surface coils. Conspicuous breast findings that fall into the BI-RADS category 4 or 5 should be verified histologically. This is preferably achieved by a percutaneous biopsy. In exceptional cases, however, primary open surgery may be an option. If a suspicious finding has a clear sonographic correlate, then ultrasound-guided percutaneous biopsy is the method of choice. If a suspicious finding has a mammographic correlate and no clear sonographic correlate (e.g., microcalcifications), then a stereotactic percutaneous vacuumassisted biopsy is usually performed. Findings detected solely on breast MRI should be histologically verified by MRI-guided vacuum-assisted biopsy. Good Practice Recommendations* Image-guided biopsies should be undertaken to obtain tissue samples from abnormalities found in the MRI BI-RADS categories 4 and 5 for histopathological verification and therapy planning. *European S 3 Guidelines: Early Breast Cancer Detection (Level of Evidence B). A major purpose of the primary performance of percutaneous breast biopsy of findings suspicious for malignancy is to reduce the number of unnecessary open biopsies (diagnostic excisions). In cases when a benign histological result is attained (without uncertain biological potential), open biopsy can be avoided and a follow-up imaging examination in 6 months is initiated. When malignancy is confirmed, it is possible to offer appropriate patient information about the necessary therapeutic consequences. In addition, surgery is planned taking oncological aspects into consideration. In this context, it is also possible to arrange for sentinel lymph node biopsy (SLNB). Good Practice Recommendations* When image-guided percutaneous biopsy yields a benign histopathological result, a short-term follow-up examination (6 months) using the corresponding imaging modality should be initiated. *European S 3 Guidelines: Early Breast Cancer Detection (Grade of Recommendation A). Recommendations: Equipment for MRI-Guided Vacuum-Assisted Interventions* *German Radiological Society Breast Diagnostics Working Group (2007). In accordance with good practice recommendations, MRI-guided biopsies should preferably be performed as vacuum-assisted biopsies using coaxial technique. Fine-needle biopsies are obsolete. Large-core biopsies should only be performed in justified exceptions. Depending upon needle size, 6–12 tissue samples are obtained during a vacuum-assisted biopsy. The average volume of the samples obtained is ~1 cm3. Several MRI-compatible vacuum-assisted biopsy systems are currently available. These differ only slightly from one another (Fig. 16.4). Good Practice Recommendations* MRI-guided biopsies should be performed using vacuumassisted technique. Fig. 16.4a–c MRI-compatible vacuum-assisted biopsy systems. a ATEC breast biopsy system (Suros Surgical Systems, Inc., Indianapolis, IN, USA). b Mammotome biopsy system (Ethicon Endo-Surgery, Cincinnati, OH, USA). c Vacora VAB system (Bard Biopsy Systems, Tempe, AR, USA). MRI-Guided Percutaneous Biopsy* – Was the target finding reproducible? (yes/no) – Position of target finding (quadrant, clock time position, distance from the skin) – Vacuum needle caliber (e.g., 11G, 9G) – Number of tissue cylinders primarily harvested (if applicable, number harvested secondarily) – Documentation of relevant complications – MRI subtraction image of target finding (Figs. 16.5a, 16.6a, 16.7a) – MRI prefire image documentation of coaxial introduction needle position (Fig. 16.7b) – MRI image documentation of biopsy cavity or of vacuumassisted biopsy needle position after biopsy (Figs. 16.5b, 16.6b) – Optional: MRI check with additional contrast administration – Optional: Mammography in CC and ML projections – Complete or partial removal of target lesion to be histologically verified by MRI-guided percutaneous biopsy – Compatibility of histology and target lesion MRI image – B1 or B2: Short-term MRI follow-up in 6 months (in special cases earlier) – B3 or B4: Interdisciplinary conference to decide on the course of action (e.g., MRI follow-up, rebiopsy, surgery) – B5a or B5b: Initiation of appropriate therapy *Recommendations of the German Radiological Society Breast Diagnostics Working Group 2007.
Special Considerations in MRI-Guided Interventions
MRI-Compatible Surface Coils for Breast Interventions
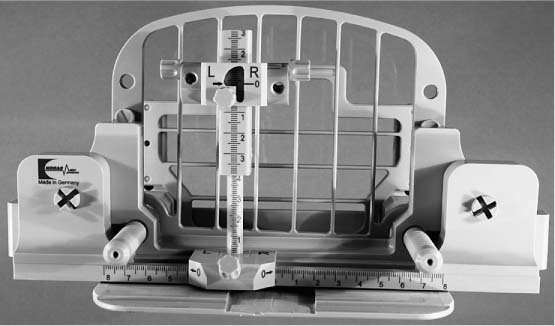
 The post-and-pillar targeting equipment (Fig. 16.2), allowing continuous adjustment of the x– and y-axes
The post-and-pillar targeting equipment (Fig. 16.2), allowing continuous adjustment of the x– and y-axes
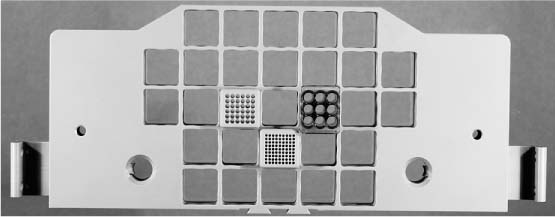
 The biopsy and localization grid (Fig. 16.3), a grid system with flexible positioning of insertable guide blocks
The biopsy and localization grid (Fig. 16.3), a grid system with flexible positioning of insertable guide blocks
Computer-Aided Calculation of Puncture Coordinates
Diagnostic MRI-Guided Vacuum-Assisted Biopsy
Indications and Objectives
 MRI system of at least 1.0 T
MRI system of at least 1.0 T
 Open surface coil allowing access to the breast
Open surface coil allowing access to the breast
 MRI-compatible localization device
MRI-compatible localization device
 MRI-compatible biopsy device: vacuum-assisted biopsy (method of choice) or large-core biopsy (when justified)
MRI-compatible biopsy device: vacuum-assisted biopsy (method of choice) or large-core biopsy (when justified)
Biopsy Equipment
 Contrast-enhanced breast MRI is performed with the localization equipment positioned on the affected side. An external marker (e.g., oil-containing) is placed at the estimated puncture site.
Contrast-enhanced breast MRI is performed with the localization equipment positioned on the affected side. An external marker (e.g., oil-containing) is placed at the estimated puncture site.
 If the target lesion is not or is only questionably reproduced, then the intervention should be discontinued and a short-term followup examination in 6 months recommended.
If the target lesion is not or is only questionably reproduced, then the intervention should be discontinued and a short-term followup examination in 6 months recommended.
 If the target lesion is clearly identified, then the puncture coordinates (x-, y-, and z-axes) are calculated. The external marker is moved to the calculated puncture site and an MRI check is performed without additional contrast administration.
If the target lesion is clearly identified, then the puncture coordinates (x-, y-, and z-axes) are calculated. The external marker is moved to the calculated puncture site and an MRI check is performed without additional contrast administration.
 If the marker position is incorrect, a new calculation is done and the marker position is corrected. If the marker position is correct, the skin is disinfected and local anesthesia is administered (if necessary, skin nick incision). The coaxial introduction needle is positioned (e.g., using the metal puncture stylet) to the appropriate depth (z-axis). The correct depth in relation to the lesion depends on the vacuum-assisted biopsy system being used.
If the marker position is incorrect, a new calculation is done and the marker position is corrected. If the marker position is correct, the skin is disinfected and local anesthesia is administered (if necessary, skin nick incision). The coaxial introduction needle is positioned (e.g., using the metal puncture stylet) to the appropriate depth (z-axis). The correct depth in relation to the lesion depends on the vacuum-assisted biopsy system being used.
 The puncture stylet is replaced by an MRI-compatible mandrin and the position of the coaxial introduction needle is checked by performing a T1w MRI series without contrast. If the position is incorrect, a new calculation is performed and the needle is repositioned and rechecked. If the position is correct, the MRIcompatible mandrin is removed and replaced with the vacuumassisted biopsy needle.
The puncture stylet is replaced by an MRI-compatible mandrin and the position of the coaxial introduction needle is checked by performing a T1w MRI series without contrast. If the position is incorrect, a new calculation is performed and the needle is repositioned and rechecked. If the position is correct, the MRIcompatible mandrin is removed and replaced with the vacuumassisted biopsy needle.
 Contiguous tissue cylinders are then harvested using the vacuum- assisted biopsy needle by rotating the biopsy notch. It is recommended to obtain ≥ 12 tissue cylinders, which is the equivalent of one complete rotation around the position “clock” (1-o’clock to 12-o’clock positions). When appropriate, an additional MRI check can be performed before ending the intervention to confirm the representative position of the biopsy cavity or to redirect further sampling.
Contiguous tissue cylinders are then harvested using the vacuum- assisted biopsy needle by rotating the biopsy notch. It is recommended to obtain ≥ 12 tissue cylinders, which is the equivalent of one complete rotation around the position “clock” (1-o’clock to 12-o’clock positions). When appropriate, an additional MRI check can be performed before ending the intervention to confirm the representative position of the biopsy cavity or to redirect further sampling.
 Final documentation of representative tissue sampling (target size is reduced, target is no longer detected, the biopsy cavity position is correct) in postinterventional MRI check (when indicated with contrast administration, e.g., mass lesions). If necessary, further sampling should be performed.
Final documentation of representative tissue sampling (target size is reduced, target is no longer detected, the biopsy cavity position is correct) in postinterventional MRI check (when indicated with contrast administration, e.g., mass lesions). If necessary, further sampling should be performed.
 Optional placement of a marker coil/clip in the biopsy cavity through the coaxial introduction cannula. MRI documentation (without contrast) of intramammary coil position.
Optional placement of a marker coil/clip in the biopsy cavity through the coaxial introduction cannula. MRI documentation (without contrast) of intramammary coil position.
 Removal of the coaxial introduction needle. Compression and cooling of puncture channel. Compression bandage.
Removal of the coaxial introduction needle. Compression and cooling of puncture channel. Compression bandage.
 Optional performance of postbiopsy mammograms in CC and ML projections for the topographic localization of the biopsy cavity, or to document the coil position in case surgery is indicated.
Optional performance of postbiopsy mammograms in CC and ML projections for the topographic localization of the biopsy cavity, or to document the coil position in case surgery is indicated.
 Report:
Report:
 Documentation:
Documentation:
 Quality criteria:
Quality criteria:
 Postbiopsy procedure depending on the reported category B pathology (Table 16.1):
Postbiopsy procedure depending on the reported category B pathology (Table 16.1):
MRI-Guided Interventions
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree